Radiation: Everyday radiochemical laboratory

In this article I will return to the topic of radiation covered in my post about the Geiger counter.
... In the late eighties, early nineties, people often went to the market with a dosimeter, choosing with the help of it “clean”, as they thought, vegetables and fruits. Sometimes and now in thematic public forums and forums there is a question: which dosimeter to buy in order to go to the market to buy products. And if there are competent people in the community, they will give the correct answer: no. And they will explain that the dosimeter detects the radioactivity of food only at levels that exceed the limit many times, the dosimeter does not distinguish the harmless activity of potassium-40 from the dosimeter equivalent, but deadly with regular consumption of strontium-90, and alpha-active and highly radiotoxic plutonium America does not even see, and to assess the suitability of the product for use, research is needed in a special laboratory.
At the moment I am working in such a laboratory. We do not do sanitary measurements. Our task is to study the radioactivity of the natural environment - mainly sea water and sediments. We are not interested in the fact that standards are exceeded, but the levels of radionuclides in natural objects themselves, the forms in which they are present, their distribution and migration. Fortunately, while the content of radionuclides in the environment in most cases is very small. And I would like to tell you how we find these low levels, and at the same time dispel some common myths.
On KDPV - Novaya Zemlya, where I visited the year before last as part of an expedition aboard the research vessel Mstislav Keldysh to the Arctic.
Alpha, Beta, Gamma, Cribble, Crab, Booms
A unique property of radioactive decay, as a source of analytical signal, is that we easily register a single decay event - that is, what happened with one atom. Therefore, the measurement of radioactivity often exceeds the sensitivity of any other analytical methods. Only very long-lived elements — uranium-238 and 235, thorium, and sometimes neptunium — are more sensitively determined chemically.
As everyone probably knows, during radioactive decay, alpha particles are emitted — helium-4 nuclei, beta particles — electrons and sometimes positrons, gamma-quanta, and in rare cases — neutrons, fragmentation nuclei and protons. Sometimes, however, it happens that nothing seems to be emitted: on the contrary, the nucleus captures an electron. But even in this case, it cannot do without radiation: the electron shell of an atom, rearranging itself, emits characteristic X-rays.
It is easiest if the isotope of interest is a gamma emitter. Gamma radiation rarely exists separately from all the others - only when the long-lived nuclear isomers transition to the ground state of the nucleus. As a rule, it occurs during alpha and beta decay, due to the fact that after the collapse of a new nucleus, you need to lose excess energy. Due to its penetrating power, gamma radiation usually easily leaves the very thick sample, which is impossible in the case of alpha radiation and is not always possible when it comes to beta. Moreover, gamma radiation has a good feature: its spectrum is ruled, and it uniquely identifies the nuclide that emitted it.
Alas, not all radionuclides are effective sources of gamma radiation. Someone has a gamma-ray emitted in 0.0001% of all decays, someone has a decay all at once into the ground state of the daughter nucleus and you will not get any gamma from it. Therefore, you have to look at alpha and beta radiation.
From school we know that alpha radiation is delayed by a piece of paper. I will say more: it is delayed by a couple of centimeters of air, and most importantly - it is delayed by the sample itself. And if we try to detect alpha radiation by bringing a sensor to it outside, then only alpha particles emitted by the uppermost layer of the substance, a fraction of a micron or micron unit, will fall into it. A similar problem with the registration of beta radiation. If it is tough (like strontium-90), it is able to overcome several millimeters of sample. And tritium beta rays are even less “punched” than alpha particles, and they cannot overcome any window. Even carbon-14 or nickel-63 beta particles hardly pass through the thin mica of a Geiger counter or an opaque foil covering a scintillation detector.
Then I will tell you what they do with this impenetrability and how they cope with it.
But first - about gamma spectrometry
Pro gamma spectrometry is most certainly mentioned in any discussion on the subject of “checking fungi dosimeters”. This is understandable: the method within the framework of solving the problem “to determine cesium-137 at the level of the MPC” is relatively simple instrumental (up to the home “knee” options) and quite express (that is, it gives a quick result).
Gamma-spectrometry is based on the fact that the gamma-radiation that occurs during the radioactive decay of this particular isotope is a stream of practically monoenergetic gamma quanta. That is, on the emission spectrum we see a narrow line, or several lines. And this spectrum is characteristic, it is possible to reliably identify the radionuclide.
If optical radiation or even X-rays can be decomposed into a spectrum using some kind of dispersing element - a prism or a diffraction grating (for X-rays, the latter is a crystal lattice, for example, graphite), then the only way to get a gamma-radiation spectrum is to measure the energy of each of the recorded its quanta. There are many ways to do this, for example, there are various ways in which a gamma quantum is “converted” into an electron with almost the same energy, and then the flow of electrons is expanded into an energy spectrum in a magnetic field. But such methods are applicable in experimental nuclear physics - but not in routine measurements. Usually, a proportional ionizing radiation detector serves to measure the energy of gamma rays.
A Geiger-Muller counter, for example, is not such a detector. Having absorbed the gamma radiation of americium-241, it will form a pulse that will not differ from the same pulse that the Geiger counter will give in response to the gamma radiation quantum of cobalt-60, despite the fact that the energies of these two quanta differ in 23 times But the scintillation counter, on the contrary, has the property of proportionality - the intensity of the flash of light, and hence the amplitude of the pulse at the anode of the photomultiplier tube is determined by the amount of energy absorbed in the crystal.
A scintillation gamma spectrometer, therefore, is simply a scintillation detector — a crystal of a scintillator, such as sodium iodide, activated by thallium, to which a PMT is attached. Pulses with PMT are fed to a special device called a multichannel analyzer (often the English abbreviation MCA). In fact, this is an ADC, but with a number of specific requirements (in particular, extremely small differential nonlinearity, which in normal applications few people care about). The principle of its operation is that it measures the value (its amplitude, or the integral under this impulse) of each impulse and “expands” these impulses in “piles” in accordance with their size. These "piles" of channels are usually from 256 to 4096 and more. In essence, MCA works like a function called with every new impulse:
unsigned int spectrum[4096] = {0}; // В этот массив набирается спектр void mca(unsigned int magnitude) // С каждым новым импульсом измеряем его { // амплитуду в интервале от 0 до 4095 spectrum[magnitude]++; // и вызываем вот эту функцию, которая инкре- return; // ментирует соответствующий элемент массива. } And then, when there are a lot of pulses, you can build a graph, which becomes a visual display of the gamma spectrum. Something like this:
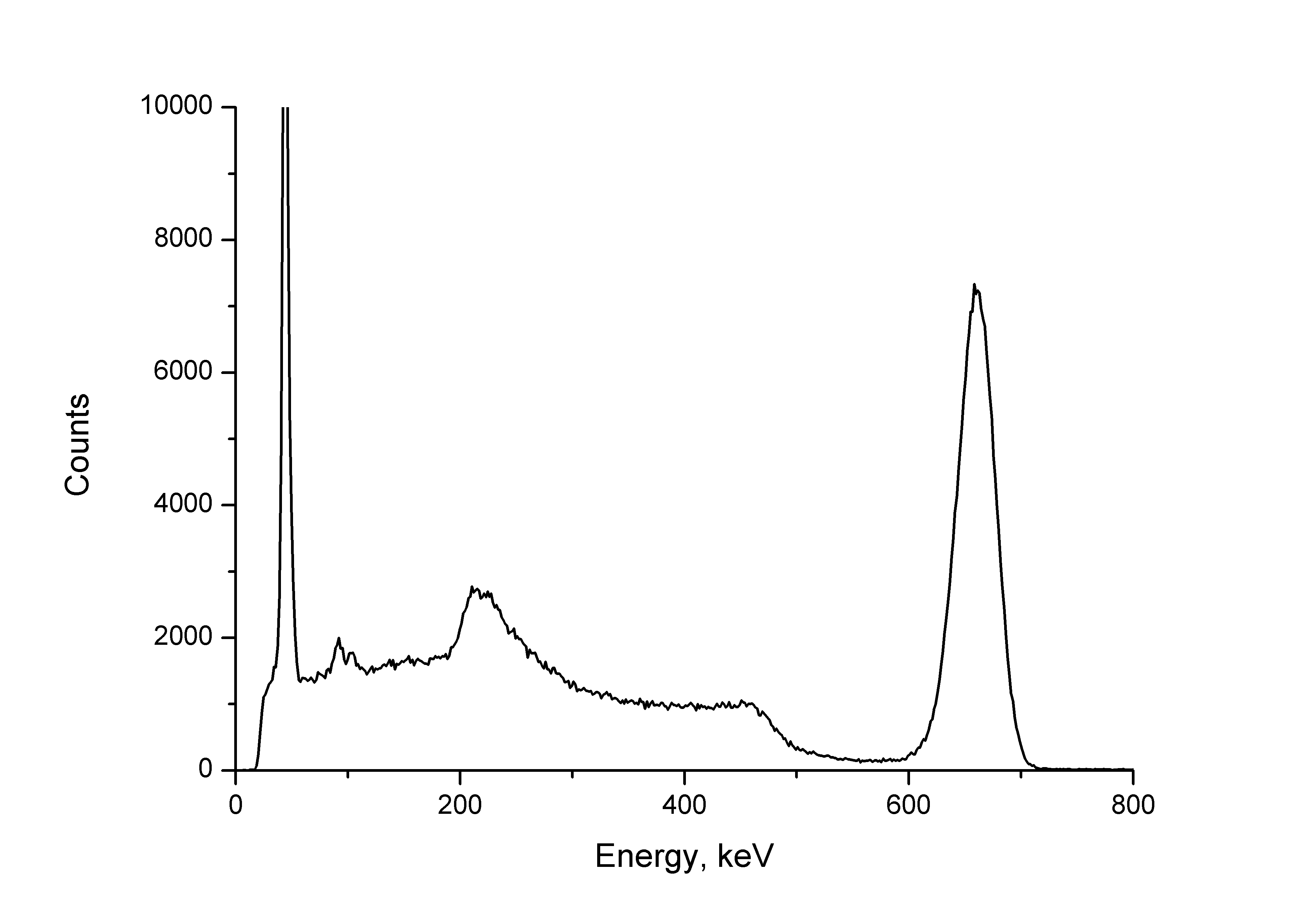
This I gave a very illustrative picture, which demonstrates that everything seems to be simple, but at the same time - not very. The fact is that this is a spectrum recorded from a source of monoenergy radiation. But it is not the only “stick” at 662 keV. Not only that instead of a stick, we have a rather vague “bell”. To the left of it, we have what actually is not (except for the leftmost peak of the peak - it exists in reality). Alas, the instrumental spectrum is not equal to the real one.
Where do these differences come from? From the physics of the gamma-radiation registration process.
The gamma quantum can be absorbed in the scintillator crystal entirely, giving it all the energy that turns into the kinetic energy of the photoelectrons, which ultimately excite a luminescence flash in the crystal - scintillation. From such quanta we have a peak on the right, we call it a photo stick , because it refers to absorption through the photoelectric effect. And another quantum can “go through”, giving it only part of the energy. Moreover - any: from almost zero to a certain limiting share - depending on the angle at which the electron interacting with the quantum will fly away. This is the Compton effect. And from him - this is a wide plateau to the left of the peak - the Compton continuum . At high energies, we will also see such an effect as the formation of electron-positron pairs, due to which single and double emission peaks appear on the spectrum, which are 511 and 1022 keV down from the photopeak, and the 511 keV peak itself from gamma radiation annihilation. Against the background of the Common continuum, a backscatter peak is seen - this is the reflected gamma radiation from the surrounding objects detector, due to the Compton effect, which has lost some of the energy, and even lower we see the characteristic X-ray lines from lead protection. But the leftmost line is also a characteristic X-ray line, only from the barium that cesium has turned into, having broken up. Yes, it is a spectrum of cesium-137. And almost everything that we see on this spectrum is a display of a single spectral line . There will be two lines - each will have the same appearance, and we will see their sum. And yes - the appearance of each of these lines depends on its energy: with its growth, the fraction of the Compton component first increases and the photo peak drops, then the effects of the production of electron-positron pairs appear (emission peaks, annihilation peak). From here we get a decent complexity of processing spectra.
Scintillation gamma spectrometer - the device, as I said, is relatively simple. Up to the point that any housewife can get it. In all seriousness: devices worth less than a thousand dollars are produced and sold, which all you need for work is a computer with a USB port and lead protection. Inside the cylindrical body - everything, and the crystal, and the photomultiplier, and its power source, and ADC. Interested - google about Atom Spectra. And those who know how to hold a soldering iron in their hands are quite capable of making such a device on their own - the sound card of a computer and a special program, for example, BeckMoni , can play the role of a multichannel analyzer, or it can be based on a microcontroller, an integrator with a reset and an external ADC (the built-in has very bad options) make the MCA, not inferior to what makes the same "Greenstar". Yes, and laboratory instruments sometimes fit into the price tag of "up to a million rubles" and (not counting lead protection) almost do not occupy space on the laboratory table (for example, Green Star's Kolibri has a size of 8x13x3 cm and also works from a USB port). They have one drawback - low resolution.
The best NaI (Tl) crystals give a spectral resolution along the cesium-137 line of about 6%. New and very expensive scintillator - lanthanum bromide is 3.2%. And these numbers lead to the fact that the real spectrum looks like this:
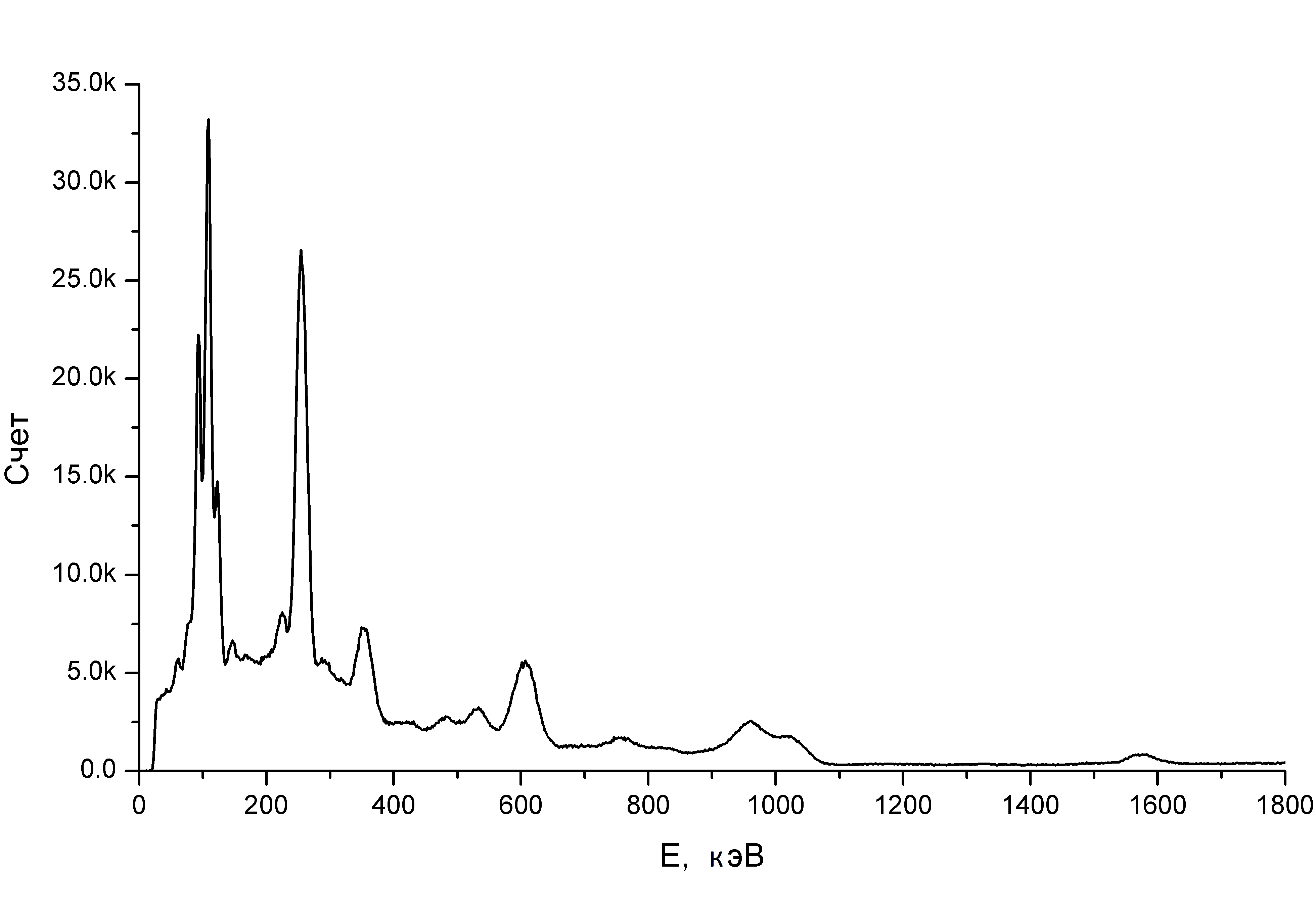
And at worst - it will be such an inexpressive hill, on the slope of which there are barely visible individual hillocks, through which isotopes can be identified somehow, but their quantitative definition is not mentioned. And our natural samples on a scintillation gamma spectrometer look like that. And the spectrum “at best”, by the way, is from a pebble, from which “Terra-P” was filled with a trill and showed milli-tergens per hour (it would give almost the same picture to a granite, but it would take a whole day to wait for a set of spectrum, plucked in a minute).
Therefore, in most cases we work on a spectrometer with a semiconductor detector. By design, it resembles a germanium pin photodiode, hidden from the light, but accessible to gamma rays. And in fact - it's just an ionization chamber. Only it was filled not with gas, but with undoped germanium, to which contacts were made in the form of a p-region on the one hand and an n-region on the other. A photon that has flown through the detector (or rather, photon generates on its way electron-hole pairs that are pulled by the electric field from the voltage applied to the semiconductor crystal to the electrodes of this ionization chamber, which results in a short and very weak current pulse, again proportional to the energy, due to the very low energy required for pairing, and for a number of other reasons, the spectral resolution of the OFG or HPGE detector is tenths of a pro cient and spectral line in the spectrum really -. line (although her companions as Compton continuum departure peaks, backscatter, and other things - is not going away).
To illustrate - this is not the spectrum of my work and I took it from the Internet . This is a total spectrum of 89 samples of salmon caught off the coast of British Columbia, showing that the Fukushima echo did not reach there: traces of cesium-137 were found, but there is no “fresh” cesium-134 with a short half-life.
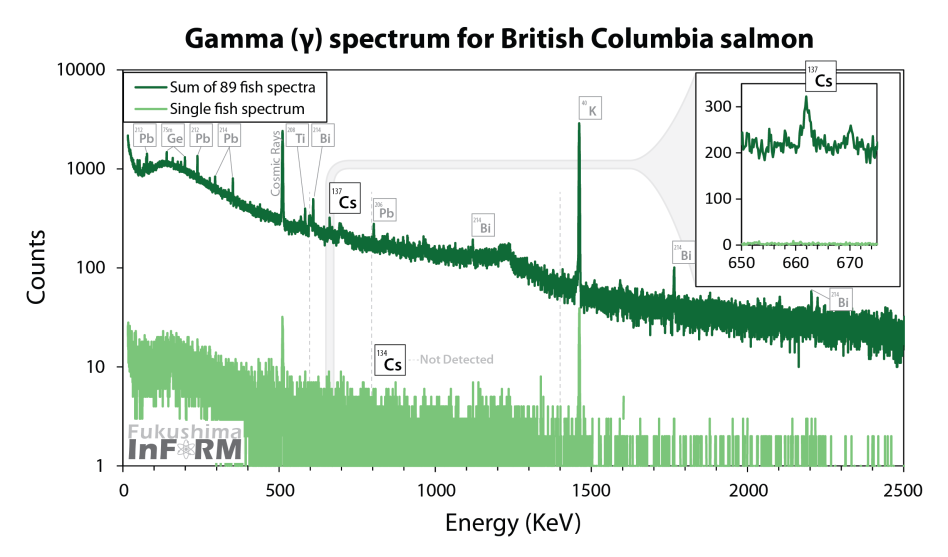
See how all sorts of weak and small lines appear on the spectrum? Scintillation gamma spectrum would not give here absolutely nothing. First of all, because the cesium-137 line would superimpose on the 609 keV line, related to bismuth-214, and the cesium-134 line would not even try to separate with the lead-214 line.
But PPD does not work at room temperature and requires cooling with liquid nitrogen, and in general it is a very expensive device, which is not in every laboratory. We were lucky - we have our own "Canberra", but we still measure some of the samples at the Department of Radiochemistry of the Chemistry Department of the Moscow State University.
But the scintillation spectrometer can be taken along on the ship. And even put in a bag and set the spectrum of the gamma background on Novaya Zemlya during the landing.
Alpha spectrometry and a little bit about beta rays
Alpha radiation of radioactive isotopes is also monoenergetic and its spectrum is characteristic. Therefore, alpha-spectrometry is a very valuable source of information about the radionuclide composition. And in some ways it is a simpler method than gamma spectrometry: the alpha particle is always absorbed completely in the detector, therefore the instrumental spectrum of alpha radiation coincides with the real one, taking into account the limited spectral resolution. Yes, and the detector is simple, like a penny: it is either a thin scintillator, or the same semiconductor detector, which is very similar in structure to alpha radiation to a standard pin photodiode, with the only difference being that the thickness of the "dead" layer surfaces including the metal and the p + region have the smallest possible thickness (remember the penetration ability of alpha particles). It does not need cooling, and since alpha particles have energies of several MeV, electron-hole pairs with each of them go out a lot and the signal level is not as low as with HPGE, where it is necessary to use a very low-noise preamplifier cooled with the detector.
Difficulties here arise only because of the same low penetrating power. The detector, together with the sample, is placed in a small vacuum chamber, which is pumped out to a few millimeters of mercury, and the sample is made very thin. One of the methods is electroplating — a nitrate solution containing alpha-active isotopes is placed in an electrolysis cell, the anode is a platinum wire, and the cathode is a stainless steel disk. Pre-solution to the maximum clear of all the excess with the help of a column with an ion exchange resin. One and a half hours - and 10 milliliters of solution turned into a film no more than one-tenth micron thick.
As for the beta rays, their spectrum is not so bright and impressive. Due to the fact that with each beta decay a part of the energy (and what part will have to) blows off the antineutrino, the spectrum of beta radiation is continuous, it looks like wide humps. Therefore, it is often limited to their score, after selecting the element of interest by chemical means.
Here, if the radiation is sufficiently hard, it can be registered with a scintillation detector and semiconductor (similar to that for alpha radiation, but thicker - and there are also universal detectors, as in the “workhorse” of the radiochemical laboratory - the desktop alpha-beta radiometer MFI-2000). And if we got, for example, tritium, then there is no better option than to take and mix the sample with a liquid scintillator. This method is called liquid scintillation counting. By the way, it is suitable for alpha, and in general is a fairly universal method. The devices, however, are again expensive and complex, we do not have such a device, we give the samples either to the Radiochemical Laboratory of the GEOKHI RAS, or to the Department of Radiochemistry of Chimfack. The reason is, first of all, that the decay energy is often very small, so in the case of tritium it is necessary to catch light pulses that make up just a dozen or two photons. It uses the favorite method of nuclear physicists - the method of coincidence. The photomultiplier, even in the absence of light, constantly generates pulses corresponding in amplitude to one or even several photoelectrons. But the probability that the pulses exceeding the one-electron one will coincide at once with the three PMTs at the same time moment is very small. But a real outbreak of scintillation, even if there were only 10-15 photons in it, will give a coincident response across all three channels at once and will be recorded.
A few words about protection
When it comes to radiation, it does not go without talking about protection from radiation. We also have to think about this, but not to protect ourselves - the levels of exposure from our samples are extremely low. You need to protect our devices, otherwise the external radiation background will negate all attempts to see weak radiation fluxes. The smaller the background in the defense, the more sensitive the definition.
The easiest way is with alpha radiation. It itself does not pass through anything, and the energy of alpha particles differs sharply from the background gamma radiation, therefore, the protection of the alpha spectrometer is not particularly required. Gamma spectrometers and beta counters are placed in massive, usually lead protection. By the way, lead is taken special for it. "Canberra", for example, uses lead, raised from the bottom of the sea, from the shipwrecks of old ships. Firstly, there is no anthropogenic radionuclide in this lead, and secondly, lead-210 has managed to decay in it. This isotope is especially important for us, as “radioactive clocks”, which allow determining the rate of accumulation of precipitation on the seabed.
To further reduce the background, including those associated with cosmic radiation, the inside of the protection is lined with copper, cadmium, plastic. This is done in order to remove the X-ray fluorescence of lead, as well as secondary electrons.
And for especially low-background measurements, the equipment is placed in a deep basement or even a mine cut in low-level rocks. This is sometimes the only way to repeatedly reduce the level of cosmic rays that fly through dozens of centimeters of lead without delay.
What is radiochemistry
The usual situation is when the radionuclide of interest is so small that such a sample volume, which contains its minimally detectable activity, cannot be inserted into the device. Sometimes due to the size of the device, and sometimes for reasons of principle (as is the case with alpha-active isotopes: you need to turn the sample bucket into a film into micron fractions of a thickness). This is the task of concentration methods.
For example, we have cesium-137 in the air. There was still no nuclear war, Chernobyl was a long time ago, so there is little cesium-137. и меньше беккереля на кубометр. То есть, в вашей комнате распад одного атома цезия-137 происходит несколько раз за час. Для гамма-спектрометрии нужно хотя бы беккерель набрать. What to do? Берем пылесос, подключаем к нему специальный фильтр. Цезий будет в составе пыли и он на этот фильтр сядет. Прогнали через него тысяч десять кубометров воздуха, и полученную банку пыли можно засунуть в гамма-спектрометр.
Или другой вариант — для выделения того же цезия-137 из морской воды прогнать тысячу литров забортной воды через мочалку, пропитанную ферроцианидом кобальта, которая имеет свойство эффективно выделять цезий из воды.
Вы помните, как супруги Кюри добывали радий? Его соосаждали с сульфатом бария, многократно повторяя этот процесс и увеличивая концентрацию радия на каждом этапе. Примерно таким же способом — путем соосаждения, сорбции на ионообменных смолах и других сорбентах, электролиза и других методов мы концентрируем элемент, изотоп которого нас интересует, избавляясь от тех, что мешают (в том числе и своей радиоактивностью) и уменьшая объем пробы порой в миллионы раз.
Про один из методов концентрирования я уже рассказал, когда рассказывал про альфа-спектрометрию: из нескольких миллилитров азотнокислого раствора мы получили тончайшую пленку. А перед этим мы зачерпнули за бортом бочку морской воды, добавили туда хлорного железа, а затем осадили его аммиаком. Большая часть содержавшегося в воде плутония оказалась в осадке (соосаждение вообще часто используется в радиохимии — например, его используют для выделения стронция-90). Весь этот осадок вместе с небольшим количеством воды поместился в литровую бутылку, которую мы привезем на берег. А дальше уберем сначала лишнюю воду, потом растворим осадок и уберем оттуда железо с помощью одной ионообменной смолы, а потом уберем все остальное с помощью хроматографической колонки с другой ионообменной смолой, из которой плутоний пойдет в нужный момент времени. Вот так и появляются эти несколько миллилитров, из которых затем плутоний осаждается электролизом.
Была ли в XVII веке ядерная война?
Да, представьте себе — есть такая «теория», будто бы 200-300 лет назад случилась ядерная война и высокоразвитая цивилизация землян оказалась отброшенной в позднефеодальное-раннекапиталистическое общество. И она была не единственной: следы ядерного конфликта находят в древней Индии (Мохенджо-Даро), а еще общеизвестным является радиоактивность многих древних костей, что тоже является доказательством того, что ядерные взрывы гремели над древними цивилизациями.
Допустим, так и было. Что искать в качестве доказательств? Вы скажете «радиоактивное заражение» и будете неправы. Вернее, правы только частично.
Радиоактивность была и есть и безо всякой ядерной войны. Но радиоактивность от атомной бомбы — особая, в ней есть то, что позволяет отличить ее от природной безошибочно. Это особый радионуклидный состав.
Природная радиоактивность обусловлена совершенно определенными изотопами. Это калий-40, рубидий-87, уран и торий (с радиоактивными продуктами их распада) — в общем, изотопы, имеющие огромные периоды полураспада, позволившие им сохраниться еще с тех времен, когда не было ни Земли, ни Солнца. К ним добавляется немного так называемых космогенных изотопов — углерод-14, бериллий-7, натрий-22, тритий. Они образуются под действием космических лучей и постоянно воспроизводятся.
А вот радионуклиды, характерные для ядерного взрыва, совсем другие. В доядерную эру на Земле (не считая природных ядерных реакторов типа Окло) не было ни атома ни цезия-137, ни кобальта-60, ни рутения-106. Если они и возникли когда-то, во время вспышки Сверхновой, породившей вещество, из которого со временем образовались Солнце и планеты, то к нашей эпохе они бесследно исчезли. А спустя 200 лет наиболее долгоживущие из них сохранились бы. И мы бы нашли их — в виде отчетливых пиков активности в слоях донных осадков, какие мы видим сейчас в слоях 1950-60-х годов прошлого века, а также в слое 1986 года.
Мы бы их нашли и в Мохенджо-Даро, и в тех самых радиоактивных костях из каменного века. Но находим мы там лишь торий и уран. И продукты их распада — тот же радий.
Еще один миф: радиационный фон с момента открытия радиоактивности возрос в десятки раз. Вариант мифа с элементами теории заговора: чтобы это скрыть, в шестидесятых годах изымали радиометрические приборы из лабораторий и возвращали после перекалибровки.
Данный миф опровергается очень просто. С тех времен удивительно как, но в лабораторных залежах сохранились старые счетчики Гейгера в родных коробочках с паспортами. Типов МС-6, ВС-6 и т.п. И в них была вписанная от руки цифра «натурального фона». И если эти счетчики «запустить» сейчас, они выдадут практически те же значения фоновой скорости счета, что записана в паспорте.
И даже если предположить, что счетчики и паспорта тоже подменили — если бы в настоящий момент значительная доля фоновой радиации была обусловлена техногенной ее компонентой, то есть продуктами деления урана и плутония — на гамма-спектре фона мы бы имели отчетливые, возвышающиеся над всем остальным спектром, пики цезия-137 и других характерных нуклидов. Такую картину можно увидеть, если привезти гамма-спектрометр в Припять или хотя бы в некоторые районы Брянщины или Тульской области. А вот московские 8-12 мкР/ч обусловлены все теми же ураном, торием и калием, и на четверть — космическим излучением. И фона в 0,5-1 мкР/ч в Москве не было никогда.
Послесловие или еще раз про дозиметр на рынке
Предельно-допустимые уровни содержания радионуклидов в пищевых продуктах сильно различаются. Причиной различной радиотоксичности их является в первую очередь склонность к концентрированию в различных органах и тканях и к прочному закреплению в них. Так, у стронция-90, который накапливается в костях, рядом с костным мозгом и остается там почти что навечно, дозовый коэффициент более чем вдвое превышает таковой для равномерно распределяющегося по организму цезия-137. Поэтому если для цезия-137 предельно допустимой активностью для большинства продуктов являются значения 50-100 Бк/кг, то для радиостронция — вдвое меньшие. А вот для плутония-239 предельно допустимое поступление в организм измеряется в десятках беккерелей в год .
Поэтому — нет, дозиметр не поможет. И даже домашний гамма-спектрометр, который легко выявит загрязнение цезием-137 на предельно-допустимом уровне, «пропустит» загрязнение гораздо более опасными альфа-активными изотопами.
Source: https://habr.com/ru/post/438306/