Extracellular DNA, as a biomarker of aging and various pathologies
We have already told you about circulating miRNA, but there is another equally cool thing - extracellular circulating DNA (English cell-free DNA, circulating DNA). It was opened in 1948. Now interest in her has intensified, and this is actually the point.
Extracellular DNA (oncDNA) is found in several body fluids: in plasma and serum, urine, saliva, synovial, peritoneal and cerebrospinal fluid.
We will look at the features of the vCDNA in two fluids: blood and urine. It is clear that it is more convenient for diagnostics.
As it became known, vkDNA circulates in the blood as part of apoptotic bodies, microvesicles, nucleosomes, exosomes, nucleoprotein complexes with blood proteins and, presumably, in a free form.
It is believed that the sources of the appearance of cfDNA in the blood are cell death processes, maturation of red blood cells, DNA secretion by cells, as well as bacteria and viruses. Immediately, we note that the latter source, the DNA of bacteria and viruses, makes a very small contribution to the total pool of circulating DNA, and the level of exogenous DNA during infections does not exceed several dozen pcg / ml. Whereas according to modern data, the normal level of cfDNA in a healthy organism is 3-44 ng / ml in plasma and 50-100 ng / ml in serum.
A significant part of vkDNA in the body is likely to result from cell death, in the process of apoptosis, necrosis and netosis. About 100 billion cells per day are killed in the body of an adult through programmed cell death (apoptosis), resulting in the degradation of about 1 g of DNA per day.
During apoptosis, nuclear DNA is cleaved by a special enzyme DNase. After that, DNA fragments of a length of a multiple nucleosome appear (180–200 bp, bp – base pairs), which must be utilized by macrophages as part of apoptotic bodies (special packaging after apoptosis). Everyone knows that nucleotide pairs (adenine-thymine and guanine-cytosine) in our DNA are repeated three billion times. Nucleosomes are special structures in the composition of chromatin, consisting of histone proteins, on which a DNA strand is wound in one and a half turns.
Then a strange thing happens. For unknown reasons, part of the DNA of the dead cells avoids meeting with macrophages and enters the blood. At various times, experiments have been carried out confirming the apoptotic nature of vcDNA [1, 2].
When splitting cfdn plasma of healthy donors with the help of gel electrophoresis, bands corresponding to fragment lengths of 180-200 bp are detected. and lengths that are a multiple of this number, which corresponds to the internucleosomal chromatin cleavage during apoptosis. Detection of fetal DNA in the mother’s blood also favors apoptosis as a source of cDDNA [3].
Normally, necrosis makes a less significant contribution to the emergence of cfDNA, increasing its number in severe injuries. Necrotic VKDNA in plasma is found in the form of longer fragments - more than 10 thousand bp long. Using the PCR (polymerase chain reaction) method, it is possible to distinguish between long cDDNA fragments resulting from necrosis and shorter fragments due to apoptosis.
In addition to apoptosis and necrosis, there is another little-known type of cell death, which is the source of vcDNA - netosis (NETosis).
In case of non-toxicity, neutrophil cells emit special net-like structures, extracellular neutrophilic traps (NET, Neutrophil Extracellular Trap), whose task is to deactivate the pathogen that has penetrated the body: viruses, fungi and bacteria. At the same time, neutrophil dies, throwing DNA, histones, various proteins and enzymes into the extracellular space. It is known that sometimes the NET process is pathological in nature, contributing to the development of thrombosis, cardiovascular and autoimmune diseases, as well as cancer.
Recently, in 2018, it was found that with HIV infection, there is a hyperactivation of neutrophils, the mechanism of NET and netoz. As a result, there was an extensive death of immune cells captured by networks of neutrophils, CD4 + and CD8 + T cells, B cells and monocytes, and the development of concomitant cardiovascular pathologies [4].
Another potential source of ecDNA is the secretion of DNA by normal and tumor cells. As early as 1972, evidence of active DNA secretion by lymphocytes into the extracellular environment was obtained [5].
The presence of such a phenomenon as DNA extraction by cells into the extracellular environment implies the existence of special mechanisms for the transfer of DNA across the cell membrane. In the course of the research, it was found that the release of DNA from lymphocytes occurs with the active action of trypsin, pronase and plasmin against the background of the deficiency of Ca2 + and Mg2 + ions. An excess of calcium ions inhibits the release of DNA from the cell [6].
According to Russian biochemists from the Kazan State University, another likely source of ccDNA in the blood may be a completely unknown form of cytoplasmic DNA, other than nuclear and mitochondrial DNA - associated with the membrane of human diploid lymphocytes (Diploma Human Lymphocytes). ) [7].
Despite the fact that this type of DNA was discovered in 1971, it still remains unexplored, and there are few works by the same authors on it, although in PNAS and Nature.
By assumption Abramova ZI with co-authors, some part of the cDDNA can be formed from this cytoplasmic DNA, as indicated by the characteristic features of some cDDNA fragments that differ from nuclear and mitochondrial [6].
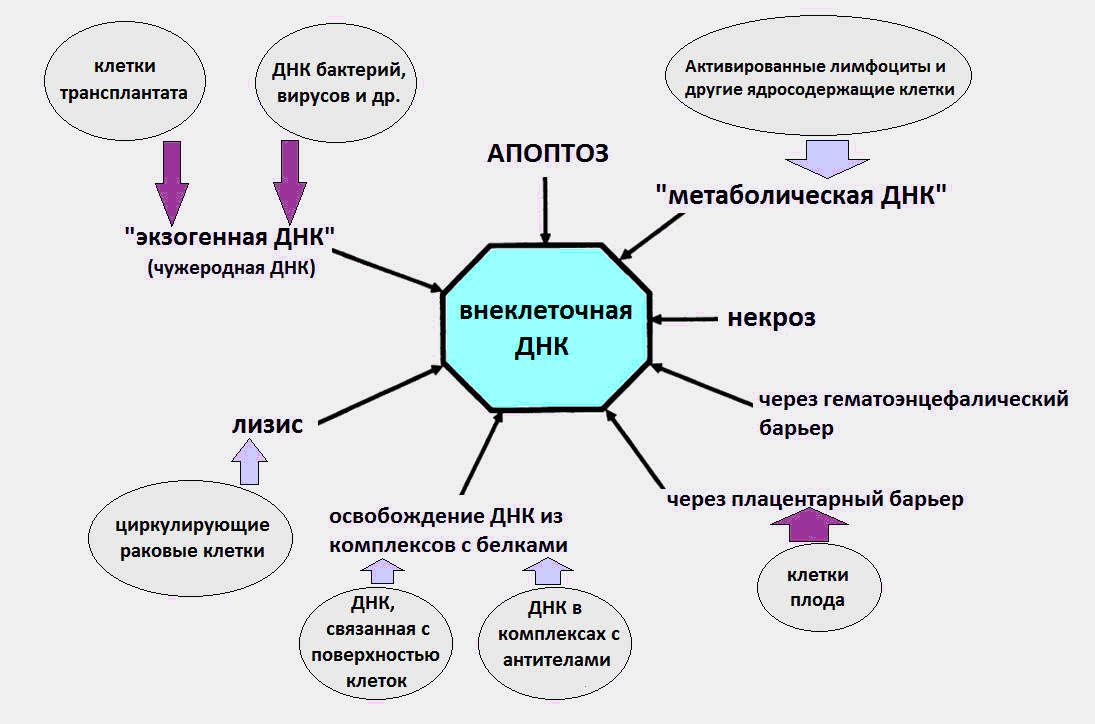
Fig.1 Various pathways by which cfDNA enters the circulation.
More details about all aspects of biology of ccDNA (immunomodulatory effects, reception of ccDNA on cell surface, etc.) can be found in the detailed review of Professor V. Kozlov. (Research Institute of Clinical Immunology, Siberian Branch of the Russian Academy of Medical Sciences) [8].
The logic dictates that the levels of the in-cDNA should change in the pathologies that are accompanied by mass cell death. A number of studies have shown a significant increase in ccDNA levels in the development of autoimmune diseases (systemic lupus erythematosus, systemic scleroderma, rheumatoid arthritis, etc.) and that there are characteristic features of cDNA typical for a specific autoimmune pathology [8-10].
It is also known that in acute myocardial infarction, which accompanies the death of heart cells of both necrosis and apoptosis, levels of cDNA also increase. And this had potential diagnostic value, since patients who developed complications had higher levels than those who recovered better [11].
In patients with acute stroke, plasma concentration of cfDNA, measured within 24 hours, correlates with the severity of stroke and may serve as a predictor of mortality and severity of outcome, even in those patients who do not have visible changes detected by neuroimaging methods [12,13] .
It is known that a large number of guanine-cytosine (GC) sequences, which are more resistant to degradation by nucleases, than adenine-thymine DNA fragments, are characteristic of vcDNA. Thus, the content of a specific GC-rich genome sequence — the transcribed region of the ribosomal repeat — in the cDNA is several times higher than the content of this repeat in nuclear DNA [14].
And in chronic pathologies involving activation of cell death (ischemic heart disease, arterial hypertension, autoimmune pathologies), the content of these HZ-rich marker sequences in the blood cDNA is 10 or more times [15]. This makes it possible to use the indicators of HZ-enriched ribosomal DNA as part of the cDNA as a biomarker of a chronically pathological process.
When fighting cancer, the challenge is to detect the tumor at the earliest possible stage. Here, VKDNA is of extreme interest, as a biomarker of tumor genesis.
Point mutations in tumor vcDNA were detected in the blood of patients long before the diagnosis was made [16].
About ccDNA levels in oncology, searching for ccDNA with mutations characteristic of carcinogenesis, such as KRAS, HER2, BRCA1 oncogenes, APC, PIK3CA, BRAF, etc., using mitochondrial DNA as a component of chDNA, studying characteristic genetic and epigenetic changes vkDNK, such as hypomethylation of mobile elements of Alu, in the event of tumors, the use of "integrity index" in cDNA and other aspects can be read in the works of Vasilyeva et al., Gonzalez-Masia et al. [17, 18].
It has been established that cfDNA is characterized by a much stronger degree of oxidation under the influence of reactive oxygen species (ROS) than for nuclear DNA.
Thus, the content of the well-known marker of DNA oxidation, 8-oxG, in the composition of nuclear DNA normally and in various pathologies ranges from 1 to 10 per million nucleotides, and as part of the cDNA, the content of this marker is 300 or more per million nucleotides [19].
A sharp increase in the content of 8-ochodG in cfDNA has been established in oncology and cardiovascular diseases, which are accompanied by oxidative stress, and can reach values of 3000 8-oxodG per million nucleotides [20].
In general, it is believed that the levels of 8-oxG in the composition of cDNA are a sensitive marker of total oxidative stress in the body.
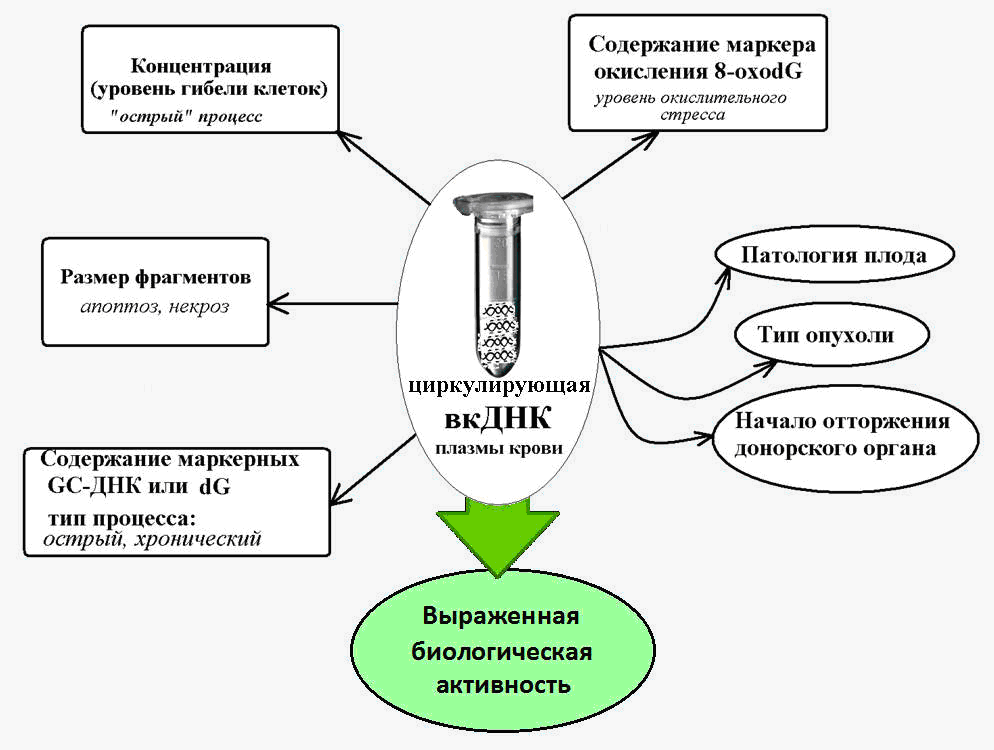
Fig. 2. Circulating extracellular DNA - a marker for various pathological conditions, accompanied by increased cell death.
A big and very important breakthrough in the research of the role of vCDNA in internal processes occurred 3 years ago. In 2015, a group of researchers made an exciting discovery that showed that ccDNA, like mobile genetic elements, is able to invade nuclear DNA and cause mutations in the genome [21]. All this is very amazing.
In earlier studies it has already been shown that tumor DNA from blood may have the ability to integrate into nuclear DNA. Mouse fibroblasts were placed in plasma taken from patients with colon cancer, after which K-ras oncogenes and p53 apoptosis p53 protein genes were detected in mouse DNA, and the cells themselves were transformed oncologically (cancer was introduced to healthy mice) [22 ]. This indicates that DNA from the plasma was absorbed and integrated by the cells placed into it. This alleged phenomenon — the absorption of oncogenes by healthy cells from circulating vcDNA in the blood — can be responsible for the process of metastasis and is called "genomestasis."
In another paper, data were provided on the uptake and integration of fragments of the DYS14 gene specific for the Y chromosome in the brain of a woman carrying a male fetus [23].
In a 2015 study, scientists traced how fluorescently labeled vcDNA taken from cancer patients and healthy people were easily incorporated into the nuclear DNA of mouse fibroblasts in in vitro experiments (that is, outside a living organism, in cell cultures). Moreover, human cfDNA was localized in the nuclei of mouse cells for several minutes, and almost 100% of the mouse cell nuclei showed fluorescent signals for 30 minutes.
Sequencing of the entire mouse cell genome revealed the presence of human DNA fragments in it, including the characteristic mobile elements of Alu. In addition, the authors found an induction of γ-H2AX-foci arising from DNA damage, as well as an increase in the activity of caspase-3 and expression of the ATM, p53, p21, GADD-34 and DNA ligase genes. That all together indicates an increased instability of the genome, activation of the response to damage to DNA and apoptosis.
Then, the authors tested the effect of the foreign cfDNA in vivo, on a living organism. The mice were intravenously injected with human cDNA, fragments of which were then found in the heart, lungs, liver and brain of mice killed 7 days after the injection.
Moreover, human DNA particles administered to mice were able to cross the blood-brain barrier and enter the brain. As in the case of cells in culture, intravenous administration of ecDNA induced induction of γ-H2AX, proapoptotic caspase-3, and activation of the response to DNA damage. And it is the response to DNA damage, according to scientists, that plays the central role of the integration of vDNA into nuclear.
Nuclear DNA perceives the extracellular insertion as a double-stranded break and starts “patching” itself, incorporating circulating DNA into its composition: “Activating the response to DNA damage combines several heterogeneous cDDNA and fragmented chromatin into long concatemers by means of non-homologous connection of the ends as part of the recovery process”.
As a result, the authors come to a paradoxical conclusion: “Being ubiquitous and constantly occurring, damage to nuclear DNA caused by extracellular circulating DNA can be the main cause of aging associated with aging abnormalities and the final death of the organism. Thus, DNA probably plays the paradoxical role of both the custodian of the foundations of life and the destroyer of life itself. ”
Needless to say, this conclusion is very much contrary to all ideas about aging today.
Logic dictates that the following dependence would correspond to this hypothesis: the longer the life span of a species, the smaller the individual of this form of DNA and / or it is less dangerous for this species.
But until such a comparative study is completed, it remains to be assumed that in the long-lived species (man) and short-lived (mouse), cDNA is produced, presumably, in relatively comparable amounts. And the detection of elevated concentrations of these molecules in the elderly may well be, on the contrary, a consequence of the aging process, and not its cause.
Although this is a consequence that can amplify the cause. Read the article Belikov.
In addition, the authors of the discovery propose to refer vcDNA to another class of mobile genetic elements. And then, perhaps, the wide participation in the evolution of such a process as horizontal gene transfer, both in invertebrates and vertebrate representatives of the animal world, will receive its explanation. Due to which, according to one of the latest data, the human genome could borrow from other species a few dozen genes [24]
A very important question remains open: how exactly do cDNA get into the cell? Existing hypotheses include assumptions that particles containing DNA can penetrate cells through membrane pores, as well as through different types of endocytosis, proteoglycan-dependent macropinocytosis, or when interacting with membrane proteins.
Another interesting discovery was made a year later by Japanese researchers. They were able to describe the active participation of cDNA, which appeared in the blood as a result of the death of adipocytes in people with obesity, in the development of inflammation and insulin resistance. A key factor interacting with these cDDNAs in these processes was the innate immunity molecule Toll-like receptor 9 (TLR9) [25].
Not without intrigue on the part of Russian scientists from the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences. They found that X-ray irradiation and the antidiabetic drug Metformin cause an increase in urine excretion of vcDNA (nuclear and mitochondrial) in rats.
Their data showed that, prior to the experiments, the amount of nuclear vkDNA was 40% higher and that of mitochondrial vkDNA was 50% higher in the urine of elderly rats compared with young ones.
12 hours after irradiation, the content of i-vcDNA and mt-vcDNA in the urine of young rats increased by 200% and 460%, respectively, compared with the control, whereas in the urine of elderly rats it increased by 250% and 720%.
6 hours after administration of metformin to other rats, the amount of i-vcDNA and mt-vcDNA in the urine of young rats was increased by 25% and 55% and by 50% and 160% in the urine of elderly rats.
According to the authors, these data suggest that X-rays and metformin cause a significant increase in cDNA in the urine of rats (especially old ones), caused by active cell death in tissues. These results also suggest that metformin may act as senolithic, initiating the death of old cells containing structural and functional impairments [26].
In October last year, scientists have traced the relationship of the levels of the ccDNA with mental stress and physical exertion in young people aged 18-36 years. The results showed a twofold increase in cDDNA after acute psychosocial laboratory stress (TSST) and a fivefold increase in cDDNA after exercise on the treadmill (with the achievement of depletion of the treadmill).
An increased amount of shorter cfDNA fragments, characteristic of apoptosis, in the blood after physical exhaustion was also recorded.
Extracellular mitochondrial DNA showed the same increase after both stress loads. In addition, the methylation profiles of the ccDNAs used in this work as a marker for various cellular origins differed significantly for different stress tests.
For the analysis of methylation, scientists chose the HOXA5 gene that is actively involved in embryonic development because of the specificity of its methylation in different cell types. So, it is characterized by hypermethylation in muscle cells, hypomethylation in brain cells, from cell lines of the hippocampus and cultured neurons, and differential methylation in blood cells.
The methylation of the HOXA5 gene in the cfDNA decreased immediately after psychosocial stress and increased after physical stress, which, according to the authors, points to various cellular sources of active release of nuclear DNA. [27].
Now the main thing. Consider the relationship of levels and specific features of cfDNA with aging.
The first works that showed such a relationship were published in 2011–13. Finnish microbiologists and immunologists from the University of Tampere studied the cDNA values in centenarians over 90 years old, participants in the Vitality 90+ study, and in young people (aged 22–37 years) as a control group.
The results of their work showed that the concentration of cfDNA in long-livers was significantly higher than in the young. There were also characteristic differences in the cDNA: in older people it was represented by more low molecular weight fragments, in young people it was more high-molecular, that is, longer.
In the following studies, the same group showed that the levels of ecdc had a clear relationship with inflammatory markers (positively correlated with the level of C-reactive protein, serum amyloid A (SAA), etc.) and mortality in people over 90 years of age. As well as the fact that higher levels of total and hypomethylated oncDNA were associated with systemic inflammation and the development of senile asthenia (frailty) [28-30].
The two most recent recent studies on ccDNA and aging last year showed characteristic epigenetic changes in this DNA.
In one of them, a team of scientists analyzed how the methylation of the mobile genetic elements LINE-1 and Alu in vDNA of healthy people, aged from 23 to 61 years, changes with age.
The LINE-1 element has a length of about 6 kbp, while the high molecular weight fragments of cfDNA, for comparison, are larger than 10 kbp.
LINE-1 refers to retroelements, i.e. to mobile elements that use reverse transcription for their reproduction, transfer of genetic information from RNA to DNA. LINE-1 is the only currently known mobile element of the genome that retains the ability to reproduce and move its copies in the genome. Also, it is the most numerous of the human moving structures: copies of LINE-1 occupy a huge proportion in DNA - one fifth of the entire genome.
The second element, Alu, cannot independently reproduce and make new insertions into the genome and depends in this process on the genetic apparatus of the element LINE-1 (for more information on retroelements of the genome, see the article in Popular Mechanics “ Retroviruses:“ fifth column ”DNA ”).
The results of the study showed that with aging there is a significant decrease in methylation of retro-elements LINE-1 and Alu, and this process was noticeably more intensive in smokers.
As established today, the activity of the elements LINE-1 and Alu, which are able to insert their copies into the genome, contribute to a significant part of human genetic diseases and cancer. More than 100 LINE-1-mediated insertions (inserts) that lead to human genetic diseases are known, and several LINE-1 inserts have been found that interrupt tumor suppressor genes and provoke cancer [31].
In the second study, scientists confirmed a decrease with age of the methylation of the elements LINE-1 and Alu in the composition of the ex-DNA. Moreover, among the 4 groups in the study (healthy long-livers over 100, unhealthy long-livers over 100 years old, elderly people (71 years old) and young people (25 years old)), healthy long-livers with young people and older people with unhealthy long-livers had similar characteristics of vcDNA:
“Constantly in our study, we noted a greater similarity in the profiles of the ecDNA, both globally and locally between young and healthy long-livers, unlike the elderly and unhealthy long-livers. Consequently, our study suggests that ccDNA profiling can be used not only as a biomarker of age, but also as a predictor of overall health. ” [32].
Thus, extracellular circulating DNA turned out to be closely related to the state of health and the processes occurring in the body during aging: oxidative stress, inflammation and age-related pathologies.
Modern research methods, such as quantitative real-time PCR, allow the use of ecDNA as a very sensitive biomarker of aging-related processes.
Studies have shown a great potential value of the analysis of cfDNA in the diagnosis of cancer, transplantation, cardiovascular and kidney diseases, fibrosis, prenatal diagnosis (during pregnancy), injuries and sepsis, as well as in sports medicine.
The data obtained that cfDNA can exhibit the properties of a mobile element and integrate into nuclear DNA, thereby affecting the development of pathologies associated with mutations and aging in general, are also of undoubted interest.
All this requires further research and clarification.
Review prepared by: Alexey Rzheshevsky and little Mikhail Batin
Extracellular DNA (oncDNA) is found in several body fluids: in plasma and serum, urine, saliva, synovial, peritoneal and cerebrospinal fluid.
We will look at the features of the vCDNA in two fluids: blood and urine. It is clear that it is more convenient for diagnostics.
Sources of vkDNA in the body
As it became known, vkDNA circulates in the blood as part of apoptotic bodies, microvesicles, nucleosomes, exosomes, nucleoprotein complexes with blood proteins and, presumably, in a free form.
It is believed that the sources of the appearance of cfDNA in the blood are cell death processes, maturation of red blood cells, DNA secretion by cells, as well as bacteria and viruses. Immediately, we note that the latter source, the DNA of bacteria and viruses, makes a very small contribution to the total pool of circulating DNA, and the level of exogenous DNA during infections does not exceed several dozen pcg / ml. Whereas according to modern data, the normal level of cfDNA in a healthy organism is 3-44 ng / ml in plasma and 50-100 ng / ml in serum.
A significant part of vkDNA in the body is likely to result from cell death, in the process of apoptosis, necrosis and netosis. About 100 billion cells per day are killed in the body of an adult through programmed cell death (apoptosis), resulting in the degradation of about 1 g of DNA per day.
During apoptosis, nuclear DNA is cleaved by a special enzyme DNase. After that, DNA fragments of a length of a multiple nucleosome appear (180–200 bp, bp – base pairs), which must be utilized by macrophages as part of apoptotic bodies (special packaging after apoptosis). Everyone knows that nucleotide pairs (adenine-thymine and guanine-cytosine) in our DNA are repeated three billion times. Nucleosomes are special structures in the composition of chromatin, consisting of histone proteins, on which a DNA strand is wound in one and a half turns.
Then a strange thing happens. For unknown reasons, part of the DNA of the dead cells avoids meeting with macrophages and enters the blood. At various times, experiments have been carried out confirming the apoptotic nature of vcDNA [1, 2].
When splitting cfdn plasma of healthy donors with the help of gel electrophoresis, bands corresponding to fragment lengths of 180-200 bp are detected. and lengths that are a multiple of this number, which corresponds to the internucleosomal chromatin cleavage during apoptosis. Detection of fetal DNA in the mother’s blood also favors apoptosis as a source of cDDNA [3].
Normally, necrosis makes a less significant contribution to the emergence of cfDNA, increasing its number in severe injuries. Necrotic VKDNA in plasma is found in the form of longer fragments - more than 10 thousand bp long. Using the PCR (polymerase chain reaction) method, it is possible to distinguish between long cDDNA fragments resulting from necrosis and shorter fragments due to apoptosis.
In addition to apoptosis and necrosis, there is another little-known type of cell death, which is the source of vcDNA - netosis (NETosis).
In case of non-toxicity, neutrophil cells emit special net-like structures, extracellular neutrophilic traps (NET, Neutrophil Extracellular Trap), whose task is to deactivate the pathogen that has penetrated the body: viruses, fungi and bacteria. At the same time, neutrophil dies, throwing DNA, histones, various proteins and enzymes into the extracellular space. It is known that sometimes the NET process is pathological in nature, contributing to the development of thrombosis, cardiovascular and autoimmune diseases, as well as cancer.
Recently, in 2018, it was found that with HIV infection, there is a hyperactivation of neutrophils, the mechanism of NET and netoz. As a result, there was an extensive death of immune cells captured by networks of neutrophils, CD4 + and CD8 + T cells, B cells and monocytes, and the development of concomitant cardiovascular pathologies [4].
Another potential source of ecDNA is the secretion of DNA by normal and tumor cells. As early as 1972, evidence of active DNA secretion by lymphocytes into the extracellular environment was obtained [5].
The presence of such a phenomenon as DNA extraction by cells into the extracellular environment implies the existence of special mechanisms for the transfer of DNA across the cell membrane. In the course of the research, it was found that the release of DNA from lymphocytes occurs with the active action of trypsin, pronase and plasmin against the background of the deficiency of Ca2 + and Mg2 + ions. An excess of calcium ions inhibits the release of DNA from the cell [6].
According to Russian biochemists from the Kazan State University, another likely source of ccDNA in the blood may be a completely unknown form of cytoplasmic DNA, other than nuclear and mitochondrial DNA - associated with the membrane of human diploid lymphocytes (Diploma Human Lymphocytes). ) [7].
Despite the fact that this type of DNA was discovered in 1971, it still remains unexplored, and there are few works by the same authors on it, although in PNAS and Nature.
By assumption Abramova ZI with co-authors, some part of the cDDNA can be formed from this cytoplasmic DNA, as indicated by the characteristic features of some cDDNA fragments that differ from nuclear and mitochondrial [6].

Fig.1 Various pathways by which cfDNA enters the circulation.
More details about all aspects of biology of ccDNA (immunomodulatory effects, reception of ccDNA on cell surface, etc.) can be found in the detailed review of Professor V. Kozlov. (Research Institute of Clinical Immunology, Siberian Branch of the Russian Academy of Medical Sciences) [8].
Extracellular DNA as a biomarker in various pathologies
The logic dictates that the levels of the in-cDNA should change in the pathologies that are accompanied by mass cell death. A number of studies have shown a significant increase in ccDNA levels in the development of autoimmune diseases (systemic lupus erythematosus, systemic scleroderma, rheumatoid arthritis, etc.) and that there are characteristic features of cDNA typical for a specific autoimmune pathology [8-10].
It is also known that in acute myocardial infarction, which accompanies the death of heart cells of both necrosis and apoptosis, levels of cDNA also increase. And this had potential diagnostic value, since patients who developed complications had higher levels than those who recovered better [11].
In patients with acute stroke, plasma concentration of cfDNA, measured within 24 hours, correlates with the severity of stroke and may serve as a predictor of mortality and severity of outcome, even in those patients who do not have visible changes detected by neuroimaging methods [12,13] .
It is known that a large number of guanine-cytosine (GC) sequences, which are more resistant to degradation by nucleases, than adenine-thymine DNA fragments, are characteristic of vcDNA. Thus, the content of a specific GC-rich genome sequence — the transcribed region of the ribosomal repeat — in the cDNA is several times higher than the content of this repeat in nuclear DNA [14].
And in chronic pathologies involving activation of cell death (ischemic heart disease, arterial hypertension, autoimmune pathologies), the content of these HZ-rich marker sequences in the blood cDNA is 10 or more times [15]. This makes it possible to use the indicators of HZ-enriched ribosomal DNA as part of the cDNA as a biomarker of a chronically pathological process.
When fighting cancer, the challenge is to detect the tumor at the earliest possible stage. Here, VKDNA is of extreme interest, as a biomarker of tumor genesis.
Point mutations in tumor vcDNA were detected in the blood of patients long before the diagnosis was made [16].
About ccDNA levels in oncology, searching for ccDNA with mutations characteristic of carcinogenesis, such as KRAS, HER2, BRCA1 oncogenes, APC, PIK3CA, BRAF, etc., using mitochondrial DNA as a component of chDNA, studying characteristic genetic and epigenetic changes vkDNK, such as hypomethylation of mobile elements of Alu, in the event of tumors, the use of "integrity index" in cDNA and other aspects can be read in the works of Vasilyeva et al., Gonzalez-Masia et al. [17, 18].
It has been established that cfDNA is characterized by a much stronger degree of oxidation under the influence of reactive oxygen species (ROS) than for nuclear DNA.
Thus, the content of the well-known marker of DNA oxidation, 8-oxG, in the composition of nuclear DNA normally and in various pathologies ranges from 1 to 10 per million nucleotides, and as part of the cDNA, the content of this marker is 300 or more per million nucleotides [19].
A sharp increase in the content of 8-ochodG in cfDNA has been established in oncology and cardiovascular diseases, which are accompanied by oxidative stress, and can reach values of 3000 8-oxodG per million nucleotides [20].
In general, it is believed that the levels of 8-oxG in the composition of cDNA are a sensitive marker of total oxidative stress in the body.

Fig. 2. Circulating extracellular DNA - a marker for various pathological conditions, accompanied by increased cell death.
Extracellular DNA as a mobile element
A big and very important breakthrough in the research of the role of vCDNA in internal processes occurred 3 years ago. In 2015, a group of researchers made an exciting discovery that showed that ccDNA, like mobile genetic elements, is able to invade nuclear DNA and cause mutations in the genome [21]. All this is very amazing.
In earlier studies it has already been shown that tumor DNA from blood may have the ability to integrate into nuclear DNA. Mouse fibroblasts were placed in plasma taken from patients with colon cancer, after which K-ras oncogenes and p53 apoptosis p53 protein genes were detected in mouse DNA, and the cells themselves were transformed oncologically (cancer was introduced to healthy mice) [22 ]. This indicates that DNA from the plasma was absorbed and integrated by the cells placed into it. This alleged phenomenon — the absorption of oncogenes by healthy cells from circulating vcDNA in the blood — can be responsible for the process of metastasis and is called "genomestasis."
In another paper, data were provided on the uptake and integration of fragments of the DYS14 gene specific for the Y chromosome in the brain of a woman carrying a male fetus [23].
In a 2015 study, scientists traced how fluorescently labeled vcDNA taken from cancer patients and healthy people were easily incorporated into the nuclear DNA of mouse fibroblasts in in vitro experiments (that is, outside a living organism, in cell cultures). Moreover, human cfDNA was localized in the nuclei of mouse cells for several minutes, and almost 100% of the mouse cell nuclei showed fluorescent signals for 30 minutes.
Sequencing of the entire mouse cell genome revealed the presence of human DNA fragments in it, including the characteristic mobile elements of Alu. In addition, the authors found an induction of γ-H2AX-foci arising from DNA damage, as well as an increase in the activity of caspase-3 and expression of the ATM, p53, p21, GADD-34 and DNA ligase genes. That all together indicates an increased instability of the genome, activation of the response to damage to DNA and apoptosis.
Then, the authors tested the effect of the foreign cfDNA in vivo, on a living organism. The mice were intravenously injected with human cDNA, fragments of which were then found in the heart, lungs, liver and brain of mice killed 7 days after the injection.
Moreover, human DNA particles administered to mice were able to cross the blood-brain barrier and enter the brain. As in the case of cells in culture, intravenous administration of ecDNA induced induction of γ-H2AX, proapoptotic caspase-3, and activation of the response to DNA damage. And it is the response to DNA damage, according to scientists, that plays the central role of the integration of vDNA into nuclear.
Nuclear DNA perceives the extracellular insertion as a double-stranded break and starts “patching” itself, incorporating circulating DNA into its composition: “Activating the response to DNA damage combines several heterogeneous cDDNA and fragmented chromatin into long concatemers by means of non-homologous connection of the ends as part of the recovery process”.
As a result, the authors come to a paradoxical conclusion: “Being ubiquitous and constantly occurring, damage to nuclear DNA caused by extracellular circulating DNA can be the main cause of aging associated with aging abnormalities and the final death of the organism. Thus, DNA probably plays the paradoxical role of both the custodian of the foundations of life and the destroyer of life itself. ”
Needless to say, this conclusion is very much contrary to all ideas about aging today.
Logic dictates that the following dependence would correspond to this hypothesis: the longer the life span of a species, the smaller the individual of this form of DNA and / or it is less dangerous for this species.
But until such a comparative study is completed, it remains to be assumed that in the long-lived species (man) and short-lived (mouse), cDNA is produced, presumably, in relatively comparable amounts. And the detection of elevated concentrations of these molecules in the elderly may well be, on the contrary, a consequence of the aging process, and not its cause.
Although this is a consequence that can amplify the cause. Read the article Belikov.
In addition, the authors of the discovery propose to refer vcDNA to another class of mobile genetic elements. And then, perhaps, the wide participation in the evolution of such a process as horizontal gene transfer, both in invertebrates and vertebrate representatives of the animal world, will receive its explanation. Due to which, according to one of the latest data, the human genome could borrow from other species a few dozen genes [24]
A very important question remains open: how exactly do cDNA get into the cell? Existing hypotheses include assumptions that particles containing DNA can penetrate cells through membrane pores, as well as through different types of endocytosis, proteoglycan-dependent macropinocytosis, or when interacting with membrane proteins.
Extracellular DNA in obesity, inflammation, X-rays and stress of various nature
Another interesting discovery was made a year later by Japanese researchers. They were able to describe the active participation of cDNA, which appeared in the blood as a result of the death of adipocytes in people with obesity, in the development of inflammation and insulin resistance. A key factor interacting with these cDDNAs in these processes was the innate immunity molecule Toll-like receptor 9 (TLR9) [25].
Not without intrigue on the part of Russian scientists from the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences. They found that X-ray irradiation and the antidiabetic drug Metformin cause an increase in urine excretion of vcDNA (nuclear and mitochondrial) in rats.
Their data showed that, prior to the experiments, the amount of nuclear vkDNA was 40% higher and that of mitochondrial vkDNA was 50% higher in the urine of elderly rats compared with young ones.
12 hours after irradiation, the content of i-vcDNA and mt-vcDNA in the urine of young rats increased by 200% and 460%, respectively, compared with the control, whereas in the urine of elderly rats it increased by 250% and 720%.
6 hours after administration of metformin to other rats, the amount of i-vcDNA and mt-vcDNA in the urine of young rats was increased by 25% and 55% and by 50% and 160% in the urine of elderly rats.
According to the authors, these data suggest that X-rays and metformin cause a significant increase in cDNA in the urine of rats (especially old ones), caused by active cell death in tissues. These results also suggest that metformin may act as senolithic, initiating the death of old cells containing structural and functional impairments [26].
In October last year, scientists have traced the relationship of the levels of the ccDNA with mental stress and physical exertion in young people aged 18-36 years. The results showed a twofold increase in cDDNA after acute psychosocial laboratory stress (TSST) and a fivefold increase in cDDNA after exercise on the treadmill (with the achievement of depletion of the treadmill).
An increased amount of shorter cfDNA fragments, characteristic of apoptosis, in the blood after physical exhaustion was also recorded.
Extracellular mitochondrial DNA showed the same increase after both stress loads. In addition, the methylation profiles of the ccDNAs used in this work as a marker for various cellular origins differed significantly for different stress tests.
For the analysis of methylation, scientists chose the HOXA5 gene that is actively involved in embryonic development because of the specificity of its methylation in different cell types. So, it is characterized by hypermethylation in muscle cells, hypomethylation in brain cells, from cell lines of the hippocampus and cultured neurons, and differential methylation in blood cells.
The methylation of the HOXA5 gene in the cfDNA decreased immediately after psychosocial stress and increased after physical stress, which, according to the authors, points to various cellular sources of active release of nuclear DNA. [27].
Extracellular DNA and Aging
Now the main thing. Consider the relationship of levels and specific features of cfDNA with aging.
The first works that showed such a relationship were published in 2011–13. Finnish microbiologists and immunologists from the University of Tampere studied the cDNA values in centenarians over 90 years old, participants in the Vitality 90+ study, and in young people (aged 22–37 years) as a control group.
The results of their work showed that the concentration of cfDNA in long-livers was significantly higher than in the young. There were also characteristic differences in the cDNA: in older people it was represented by more low molecular weight fragments, in young people it was more high-molecular, that is, longer.
In the following studies, the same group showed that the levels of ecdc had a clear relationship with inflammatory markers (positively correlated with the level of C-reactive protein, serum amyloid A (SAA), etc.) and mortality in people over 90 years of age. As well as the fact that higher levels of total and hypomethylated oncDNA were associated with systemic inflammation and the development of senile asthenia (frailty) [28-30].
The two most recent recent studies on ccDNA and aging last year showed characteristic epigenetic changes in this DNA.
In one of them, a team of scientists analyzed how the methylation of the mobile genetic elements LINE-1 and Alu in vDNA of healthy people, aged from 23 to 61 years, changes with age.
The LINE-1 element has a length of about 6 kbp, while the high molecular weight fragments of cfDNA, for comparison, are larger than 10 kbp.
LINE-1 refers to retroelements, i.e. to mobile elements that use reverse transcription for their reproduction, transfer of genetic information from RNA to DNA. LINE-1 is the only currently known mobile element of the genome that retains the ability to reproduce and move its copies in the genome. Also, it is the most numerous of the human moving structures: copies of LINE-1 occupy a huge proportion in DNA - one fifth of the entire genome.
The second element, Alu, cannot independently reproduce and make new insertions into the genome and depends in this process on the genetic apparatus of the element LINE-1 (for more information on retroelements of the genome, see the article in Popular Mechanics “ Retroviruses:“ fifth column ”DNA ”).
The results of the study showed that with aging there is a significant decrease in methylation of retro-elements LINE-1 and Alu, and this process was noticeably more intensive in smokers.
As established today, the activity of the elements LINE-1 and Alu, which are able to insert their copies into the genome, contribute to a significant part of human genetic diseases and cancer. More than 100 LINE-1-mediated insertions (inserts) that lead to human genetic diseases are known, and several LINE-1 inserts have been found that interrupt tumor suppressor genes and provoke cancer [31].
In the second study, scientists confirmed a decrease with age of the methylation of the elements LINE-1 and Alu in the composition of the ex-DNA. Moreover, among the 4 groups in the study (healthy long-livers over 100, unhealthy long-livers over 100 years old, elderly people (71 years old) and young people (25 years old)), healthy long-livers with young people and older people with unhealthy long-livers had similar characteristics of vcDNA:
“Constantly in our study, we noted a greater similarity in the profiles of the ecDNA, both globally and locally between young and healthy long-livers, unlike the elderly and unhealthy long-livers. Consequently, our study suggests that ccDNA profiling can be used not only as a biomarker of age, but also as a predictor of overall health. ” [32].
Thus, extracellular circulating DNA turned out to be closely related to the state of health and the processes occurring in the body during aging: oxidative stress, inflammation and age-related pathologies.
Modern research methods, such as quantitative real-time PCR, allow the use of ecDNA as a very sensitive biomarker of aging-related processes.
Studies have shown a great potential value of the analysis of cfDNA in the diagnosis of cancer, transplantation, cardiovascular and kidney diseases, fibrosis, prenatal diagnosis (during pregnancy), injuries and sepsis, as well as in sports medicine.
The data obtained that cfDNA can exhibit the properties of a mobile element and integrate into nuclear DNA, thereby affecting the development of pathologies associated with mutations and aging in general, are also of undoubted interest.
All this requires further research and clarification.
Review prepared by: Alexey Rzheshevsky and little Mikhail Batin
Bibliography
- Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, and Knippers R. Cancer Res. 2001 Feb 15; 61 (4): 1659-65.
- Deligezer U1, Yaman F, Erten N, Dalay N. Frequent copresence of methylated DNA and fragmented nucleosomal patients. Clin Chim Acta. 2003 Sep; 335 (1-2): 89-94.
- Bishoff FZ, Dang D., Horne C., Marquez-Do D., Brincley WR, Levis de Fatal DNA for non-invasive prenatal diagnosis and pre-natal diagnosis. J. Hum. Genet. 2003. V. 73. P. 189.
- Sivanandham R, Brocca-Cofano E, Krampe N, Falwell E, Venkatraman SMK, Ribeiro RM, Apetrei C, Pandrea. Neutrophil extracellular trap production contributes to pathogenesis in SIV-infected nonhuman primates. J Clin Invest. 2018 Nov 1; 128 (11): 5178-5183.
- Rogers JC, Boldt D., Kornfeld S. Excretion of deoxyribonuclein acid by lymphocytes stimulated with phytohemagglutinin or antigen // Proc. Nat. Acad. Sci. USA. 1972. V. 69, No 7. P. 1685–1689.
- Туаева Н.О, Абрамова З.И. Внеклеточная ДНК в кровотоке человека. 2007. Учебные записки Казанского государственного университета. Т.149, с.23-32.
- Lerner RA, Meinke W., Goldstein DA Membrane-associated DNA in the cytoplasm of diploid human lymphocytes // Proc. Nat. Acad. Sci. USA. 1971. V. 68, No 6. P. 1212–1216.
- Козлов В.А. Свободная внеклеточная ДНК в норме и при патологии. Медицинская иммунология. 2013;15(5):399-412.
- Galeazzi M, Morozzi G, Piccini M, Chen J, Bellisai F, Fineschi S, Marcolongo R. Dosage and characterization of circulating DNA: present usage and possible applications in systemic autoimmune disorders. Autoimmun Rev 2003;2:50-5.
- Leon SA, Revach M, Ehrlich GE, Adler R, Petersen V, Shapiro B. DNA in synovial fluid and the circulation of patients with arthritis. Arthritis Rheum 1981;24:1142-50.
- Destouni A, Vrettou C, Antonatos D, Chouliaras G, Traeger-Synodinos J, Patsilinakos S, et al. Cell-free DNA levels in acute myocardial infarction patients during hospitalization. Acta Cardiol 2009;64:51-7.
- Rainer TH, Wong LKS, Lam W, Yuen E, Lam NYL, Metreweli C, Lo YMD. Prognostic Use of Circulating Plasma Nucleic Acid Concentrations in Patients with Acute Stroke. Clin Chem 2003; 49:562-9.
- Lam NY, Rainer TH, Wong LK, Lam W, Lo YM. Plasma DNA as a prognostic marker for stroke patients with negative neuroimaging within the first 24 h of symptom onset. Resuscitation 2006; 68:71-8.
- Вейко Н.Н., Булычева Н.В., Рогинко О.А., Вейко Р.В., Ершова Е.С., Коздоба О.А., Кузьмин В.А., Виноградов А.М., Юдин А.А., Сперанский А.И., Фрагменты транскрибируемой области рибосомного повтора в составе внеклеточной ДНК — маркер гибели клеток организма, Биомедицинская химия, 2008, том: 54(1), 78-93.
- Bulicheva, N. et al. Effect of cell-free DNA of patients with cardiomyopathy and rDNA on the frequency of contraction of electrically paced neonatal rat ventricular myocytes in culture. Ann NY Acad Sci. 2008. V. 1137. P. 273-277.
- Lecomte, T. Circulating free tumor DNA and colorectal cancer/ T. Lecomte, N. Ceze, E.
- Dorval, P. Laurent-Puig // Gastroenterol Clin Biol. 2010. V. 34. № 12. P. 662- 681.
- Васильева И.Н, Беспалов В.Г. Роль внеклеточной ДНК в возникновении и развитии злокачественных опухолей и возможности её использования в диагностике. Вопросы онкологии. 2013.-N 6.-С.673-681
- Gonzalez-Masia, JA et al. Circulating nucleic acids in plasma and serum (CNAPS): applications in oncology. Onco Targets Ther. 2013. V. 8. № 6. P. 819-32.
- Ermakov, AV et al. Oxidized extracellular DNA as a stress signal in human cells. Oxid Med Cell Longev. 2013. V. 2013. P. 649-747.
- Loseva, P. et al. Extracellular DNA oxidation stimulates activation of NRF2 and reduces the
- production of ROS in human mesenchymal stem cells. Expert Opin. Biol. Th. 2012. V.12. № 1. P. 85-97.
- Mittra I, Khare NK, Raghuram GV, et al. Circulating nucleic acids damage DNA of healthy cells by integrating into their genomes. J Biosci. 2015;40(1):91–111.
- Garcia‐Olmo DC, Dominguez C, Garcia‐Arranz M, Anker P, Stroun M, Garcia‐Verdugo JM, Garcia‐Olmo D (2010) Cell‐free nucleic acids circulating in the plasma of colorectal cancer patients induce the oncogenic transformation of susceptible cultured cells. Cancer Res. 70, 560–567
- Chan W, Gurnot C, Montine TJ, Sonnen JA, Guthrie KA, Lee Nelson J (2012) Male Microchimerism in the Human Female Brain. PLoS ONE 7, e45592.
- Crisp A, Boschetti C, Perry M, et al.: Expression of multiple horizontally acquired genes is a hallmark of both vertebrate and invertebrate genomes. Genome Biol. 2015;16:50. 10.1186/s13059-015-0607-3
- Sachiko Nishimoto, Daiju Fukuda et al. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci Adv. 2016 Mar; 2(3): e1501332.
- Gaziev A, Abdullaev S, Minkabirova G, Kamenskikh K. X-rays and metformin cause increased urinary excretion of cell-free nuclear and mitochondrial DNA in aged rats. J Circ Biomark. 2016 Oct 25; 5:1849454416670782.
- Hummel EM, Hessas E, Müller S, Beiter T, Fisch M, Eibl A, Wolf OT, Giebel B, Platen P, Kumsta R, Moser DA Transl Psychiatry. 2018 Oct 29;8(1):236.
- Jylhävä J1, Kotipelto T, Raitala A, Jylhä M, Hervonen A, Hurme M. Aging is associated with quantitative and qualitative changes in circulating cell-free DNA: the Vitality 90+ study.
- Mech Ageing Dev. 2011 Jan-Feb;132(1-2):20-6.
- Jylhävä J1, Jylhä M, Lehtimäki T, Hervonen A, Hurme M. Circulating cell-free DNA is associated with mortality and inflammatory markers in nonagenarians: the Vitality 90+ Study. Exp Gerontol. 2012 May;47(5):372-8.
- Jylhävä J1, Nevalainen T, Marttila S, Jylhä M, Hervonen A, Hurme M. Characterization of the role of distinct plasma cell-free DNA species in age-associated inflammation and frailty. Aging Cell. 2013 Jun;12(3):388-97.
- Lars Erichsen,a Agnes Beermann et al. Genome-wide hypomethylation of LINE-1 and Alu retroelements in cell-free DNA of blood is an epigenetic biomarker of human aging. Saudi J Biol Sci. 2018 Sep; 25(6): 1220–1226.
- Teo YV, Capri M, Morsiani C, Pizza G, Faria AMC, Franceschi C, Neretti N. Cell-free DNA as a biomarker of aging. Aging Cell. 2019 Feb;18(1):e12890.
Source: https://habr.com/ru/post/438766/