Kill cancer: iridium, human serum albumin and some blue light

One of the most famous Marvel superheroes has always been and will always be Logan, aka Wolverine. And what feature of his body comes to mind first, apart from of course regeneration with the speed of Flush? In a word - adamanty. This rare metal has unique properties, it is almost impossible to destroy it, and recycling takes a lot of forces. This fictional substance has several equivalents in our reality, which also have very specific properties. Among them, the special attention of scientists deserves iridium. This metal can hardly make a super-hero out of the common man, but he knows how to destroy cancer cells (Deadpool would not have refused this). How did scientists come to this conclusion, how effective is iridium in the fight against cancer and what is its future in oncology? We dive into the report of the research group for answers. Go.
Metal science
Iridium (Ir) is an extremely hard transition metal from the platinum group. Like fictional adamanty, iridium is very resistant to corrosion, even at a temperature of 2000 ° C. Another similarity of these two metals in their extraterrestrial origin. More precisely, iridium on our planet is very small, therefore, in high concentration it is found in places where meteorites fall.

Iridium (Ir)
Iridium is a fairly young metal in the scientific world, since it was discovered in 1803 by chemist Smithson Tennant. He worked on platinum with a mixture of nitric and hydrochloric acids, which has a very unusual name - aqua regia. And as is clear from the composition of this solution, after its use, you will not become a “drunken master”, like Jackie Chan in the same film, but rather a dead master. For the word “vodka” originally meant plain water, and only after the XIV century did it begin to be used to refer to an alcoholic beverage.
With the help of aqua regia, Mr. Tennant was able to obtain in pure form those impurities that were in platinum, namely osmium and iridium.
As mentioned earlier, iridium is very small - about 3 tons of this metal are mined per year. For comparison, silver mining according to some data exceeds the mark of 27,000 tons per year.
The basis of the study
The study is based on the method of treatment of oncological diseases (and some others also) already used in practice - photodynamic therapy (PDT). The main characters of this method are photosensitizers and light.
Photosensitizers * - substances that increase the sensitivity to light exposure in biological tissues.Sensitizers are quite picky, that is, they accumulate only in those tissues that need to be changed for further light irradiation procedure.
When light penetrates into the target tissue, a photochemical reaction occurs - molecular triplet oxygen ( 3 O 2 ) is converted to singlet. In addition, highly active radicals are formed. Together, this leads to the death of cancer cells.
Scientists cite as an example photofrin and aminolevulinic acid, as the most common photosensitizers in PDT therapy. However, in recent years, more and more attention has been paid to metals with a high luminescence coefficient, since they possess unusual and useful photochemical and photophysical properties. For example, TLD1433 (ruthenium) for PDT for treating the bladder and WST11 (palladium) for treating blood vessels.
Research results
So why not use iridium, scientists thought. But first you need a mechanism that allows you to apply this metal. The patient will not take iridium orally, as usual tablets. And here, human serum albumin ( CSA ) is connected to the work, which due to its properties and quantity (about 55% of all blood proteins) is an excellent carrier of various substances (in our case, drugs). Simply put, CSA can be used to deliver anticancer drugs to the desired area of the patient's body, as has been demonstrated in previous studies using osmium, ruthenium and palladium.
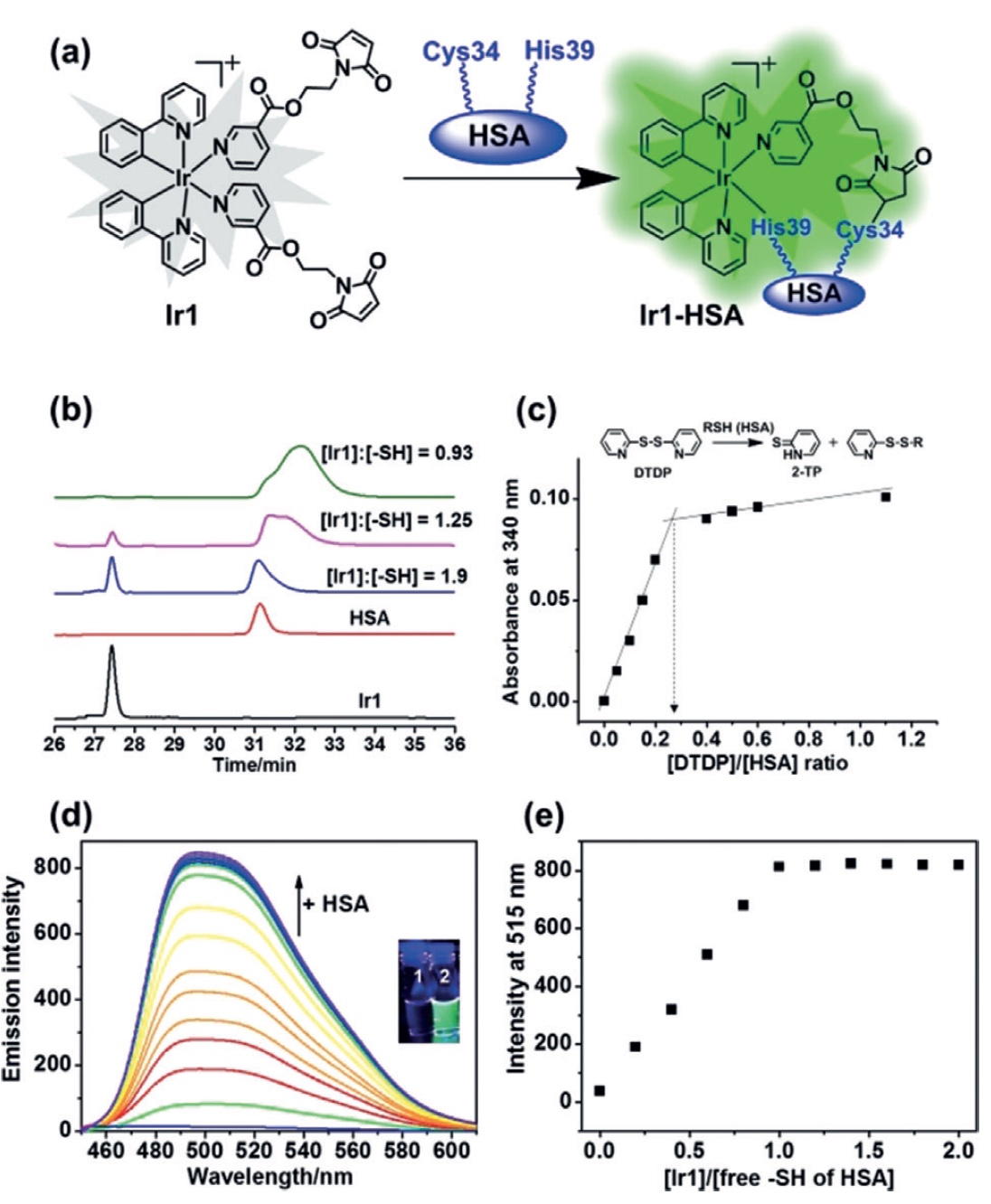
Image number 1
In the study under consideration, scientists have created a maleimide-functionalized octahedral organo-iridium (III) complex Ir1 ( 1a ) in combination with a CSA. This complex (Ir1-CSA was significantly more effective in increasing phosphorescence compared to “pure” Ir1, that is, without CSA.
In the dark, Ir1-CSA is largely non-toxic to normal cells, but exhibits strong photo-cytotoxicity towards cancer cells and their spheroids (cell formations).
Synthesized Ir1 showed stability for 12 hours in the dark and after 1 hour of irradiation with blue light. It was necessary to check the carbon-carbon bond (C = C). For this, the complex of Ir1 and cysteine (Cys) was reacted in a Cys: Ir1 molar ratio of 2: 1 in [D 6 ] DMSO / D 2 O at a temperature of 298 K for 30 minutes. As a result of proton magnetic resonance, scientists found a peak figure for vinyl protons of maleimide groups at around 6.62 ppm (a millionth part). When cysteine was added, the peaks disappeared, but then they reappeared in the 2.9 ... 3.9 ppm range. Scientists attribute this to cysteine conjugation.
Next, the scientists checked whether free thiol Cys34 from HSA is able to react with C = C. For this, 30 μM (micromoll) Ir1 were incubated with an HSA (0-120 μM) for 1 hour. Next, the resulting reaction products were separated using reverse phase high performance liquid chromatography (RP-HPLC).
When an HSA of 120 μM was reached, the Ir1 peak disappeared completely (the ratio of HSA: Ir1 = 4: 1). Thus, the content of thiol (SH) was 0.27 ± 0.1 mol SH per 1 mol HSA ( 1 s ). Therefore, the concentration of free SH-groups from 120 μM HSA is 32.4 ± 1.2 μM. With this indicator, a reaction occurs with 30 μM Ir1, leading to the appearance of an adduct (direct connection of molecules) Ir1 and HSA in a 1: 1 ratio.
Pure Ir1 did not exhibit strong radiation in an aqueous solution, in contrast to the Ir1 – HSA complex ( 1d ). The higher the concentration of CSA was, the stronger the phosphorescence of Ir1 itself became ( 1e ).
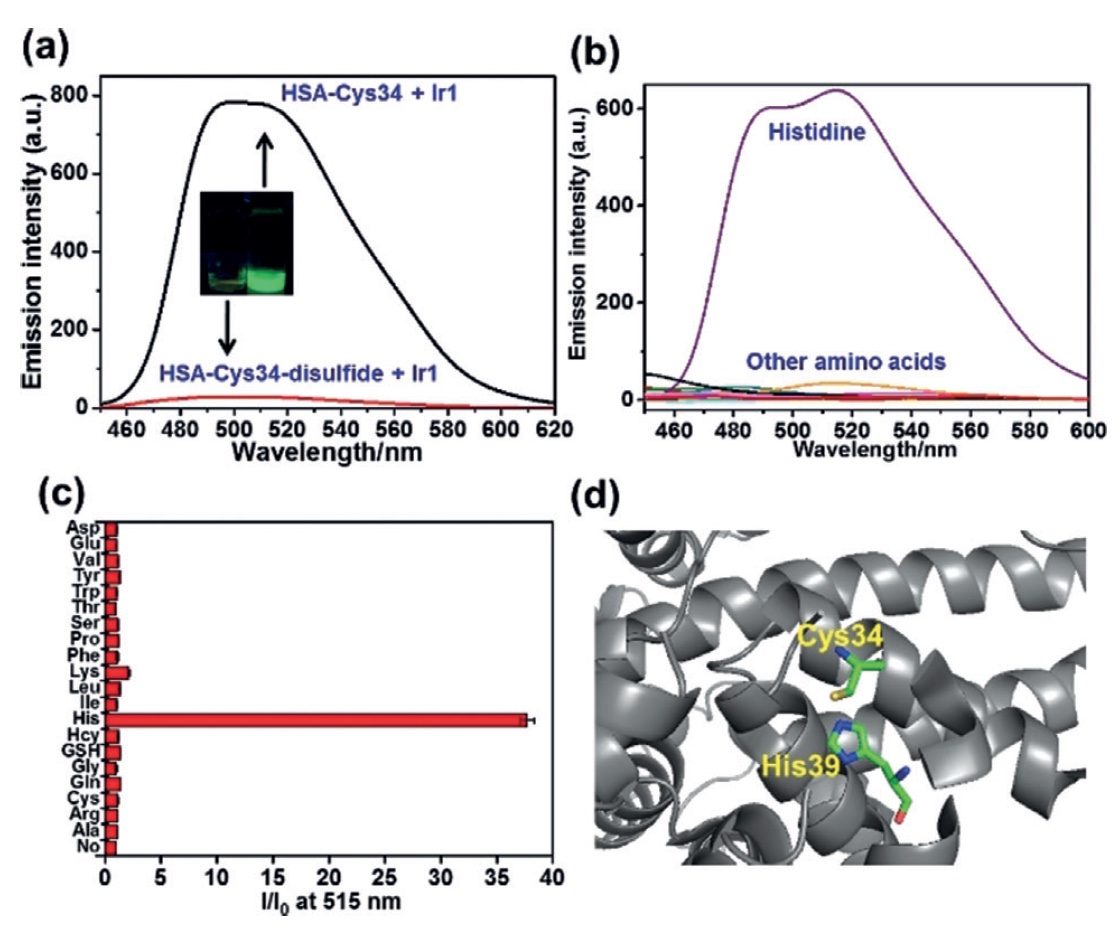
Image number 2
In order to remove free thiol groups from HSA, 100 μM of cystine was added to the solution for a day at a temperature of 277 K. The resulting product was connected to Ir1 for 30 minutes. Observations showed a significant decrease in phosphorescence. In the case of the HSA-Cys34 conjugate and Ir1 ( 2a ) conjugate, the situation was opposite, and this suggests that it is the free thiol Cys34 that is the link (or more correctly, the binding domain) for Ir1.
Now it was necessary to study the CSA in more detail by disassembling it into components. Human serum albumin has one chain of 585 amino acid residues, among which scientists need to find those that enhance the luminescence of Ir1. To this end, a luminescent analysis of the interaction of Ir1 with various amino acids ( 2b and 2c ) was carried out. And as we can see on the graph 2b , histidine (His) became the leader among the amino acids with a huge margin, increasing the luminescence of Ir1 37 times.
Having a little understanding of what and how it works inside the composite components of the Ir1-CSA complex, the scientists proceeded to practical application, that is, to experiments.
First, 0.4 millimole of Ir1 was diluted in 20 ml of MeOH: H2O, 0.4 millimole of HSA was added, and stirred for 1 hour. Further, using confocal microscopy, the distribution of Ir1-HSA in living lung cancer cells (A549) was investigated.
After only 30 minutes, the Ir1-CSA was mostly concentrated in the cytoplasm of cancer cells. At 60-120 minutes from the start of incubation, the complex penetrated into the nucleus of cancer cells.

Image No. 3: Confocal microscopy of lung cancer cells A549.
However, it is worth noting that not the whole complex penetrated into the nucleus of cancer cells, but only Ir1. An immunofluorescence test showed that CSA is simply absent in the nuclei of cells affected by Ir1.

Image No. 4: immunofluorescent analysis of the presence of CSA in lung cancer cells.
But the CSA does not disappear without a trace, it simply remains in the cytoplasm and in the membrane of the nucleus of the cancer cell. It turns out that the CSA fully performs its function: it delivered Ir to the cell nucleus, while he himself remained outside.

Image No. 5: quantum yield and lifetime of phosphorescence of Ir1 and the Ir1-CSA complex.
Scientists also checked the quantum yield (exaggerated, force) and the lifetime of the phosphorescence of Ir1 (by itself) and the Ir1-CSA complex.
The quantum yield of Ir1 was very small (only 0.001), and the lifetime at 298 K was 182.7 nanoseconds ( 5a ). But the Ir1-CSA had a quantum yield of 0.036, and the lifetime was 871.8 ns. Such a duration of phosphorescence is excellent for the generation of singlet oxygen ( 1 O 2 ).
Electron paramagnetic resonance spectroscopy using 2,2,6,6-tetramethylpiperidine as a spin trap helped detect the generation of 1O2 in pure Ir1 and in the Ir1-HSA complex at irradiation at 465 nm for 20 minutes ( 5b ). As expected, the quantum yield of 1O2 for Ir1-CSA was significantly higher (0.83) than for Ir1 (0.06).
It was also necessary to check the impact of Ir1-CSA and Ir1 on cancer cells and on healthy ones. Three options were used as cancer cells: A549 lung cancer, Hep-G2 hepatoma and cisplatin-resistant A549R lung cancer. MRC-5 (lungs) and LO2 (liver) were used as healthy cells. The experiment was conducted in two variations of illumination: total darkness throughout the experiment and blue light.
The cells were incubated with Ir1 or Ir1-CSA for 2 hours, washed with sodium perborate and irradiated with blue light for 20 minutes, or left in the dark (second experiment). After that, the cells were restored within 46 hours.
The effect of Ir1 on A549 cells both in the dark (89.6 μM) and under illumination (53.3 μM) was practically absent.
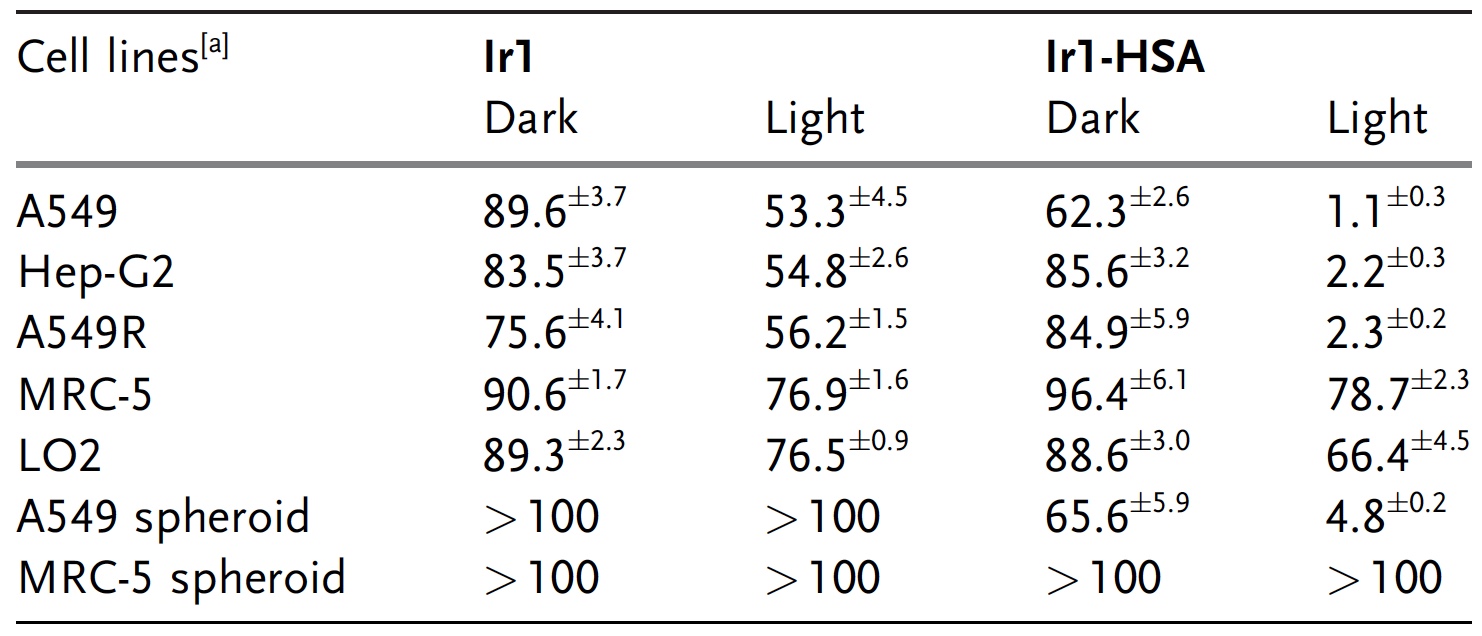
Table of the effects of Ir1 and Ir1-CSA on cancer cells: the greater the number, the less impact (i.e., more cancer cells remain intact).
But the Ir1-CSA showed completely different results. In the dark, the test complex had no effect on the cancer cells, but when illuminated, the degree of its cytotoxicity increased significantly. Similar results, as we can see from the table above, Ir1 and Ir1-HSA were also shown in relation to other cancer cells. At the same time, healthy cells in the dark and under illumination did not affect Ir1 and Ir1-CSA.
Finally, scientists analyzed the reactive oxygen species (ROS) inside the cells after light irradiation. In the dark, as expected, no ROS were detected. But in cells that were exposed to light irradiation after applying Ir1-CSA, ROS were detected ( 5c ).
For more detailed acquaintance with the study I strongly recommend to look into the report of scientists and additional materials to it.
Epilogue
According to this study, scientists did not try to invent a bicycle in the form of the use of rare metals in photodynamic therapy, because it had already been done with osmium and palladium. However, no one has yet tried using iridium, which the researchers decided to fix. Their work was not useless, as iridium showed excellent results in the fight against cancer cells of various types, while not affecting healthy ones.
Cancer is one of the most common, taking millions of lives each year. The invention of new methods of dealing with this disease and the improvement of existing ones should and will continue. Of course, we are still far from a total victory over cancer, but scientists all over the world continue their struggle in laboratories, like millions of patients in wards.
Nor should we forget the factors that lead to the occurrence of cancer. Some of them (ecology, bad habits, etc.) are completely capable of eliminating people.
Friday Offtop:
No one in their right mind would be happy with oncology. Such diagnoses make you give up and forget about everything. But you can never give up. If you do not fight, the diagnosis will definitely prevail. And if you fight the disease, there is always a chance to win over it. So why not use this chance?
Thank you for your attention, stay curious and have a great weekend, guys.
No one in their right mind would be happy with oncology. Such diagnoses make you give up and forget about everything. But you can never give up. If you do not fight, the diagnosis will definitely prevail. And if you fight the disease, there is always a chance to win over it. So why not use this chance?
Thank you for your attention, stay curious and have a great weekend, guys.
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending to friends, 30% discount for Habr users on a unique analogue of the entry-level servers that we invented for you: The whole truth about VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps from $ 20 or how to share the server? (Options are available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps until spring for free if you pay for a period of six months, you can order here .
Dell R730xd 2 times cheaper? Only we have 2 x Intel Dodeca-Core Xeon E5-2650v4 128GB DDR4 6x480GB SSD 1Gbps 100 TV from $ 249 in the Netherlands and the USA! Read about How to build an infrastructure building. class c using servers Dell R730xd E5-2650 v4 worth 9000 euros for a penny?
Source: https://habr.com/ru/post/439308/