The model of a solid nucleus without electron shells, that is, the nucleus is equal to the entire atom in size
Ilya Monin, ctn
imoninpgd@gmail.com
At present, in Physics, the Rutherford-Bohr atom model is generally recognized, in which a small heavy positively charged nucleus is surrounded by almost weightless electron shells, while the size of the electron shells is 1000 times larger than the size of the nucleus.
The Rutherford-Bohr atom model has many inherent flaws that have been hushed up for 100 years since its creation.
The only thing that explains the model of the Rutherford-Bohr atom is the experience of dispersing the flow of alpha particles on a thin golden foil. From which a conclusion was drawn about the vanishingly small size of the positive nucleus in the composition of a huge atom filled with the emptiness of the electron shells.
And how does the Rutherford model explain the existence of solids with fixed density?
But she does not explain any properties of real substances, known to us by everyday life.
It was for this reason that I had to compose my own model of the structure of the atom.
None of the existing models can explain everything at once.
I in my model do not explain the dispersion of alpha particles in Rutherford’s experience.
Well, the Rutherford model explains only one experiment on the dispersion of alpha particles on gold foil, but is unable to explain anything else.
But my model explains the existence of solids, liquids, gases and all phase transitions between them.
In the Rutherford-Bohr model, it is completely incomprehensible on what forces there are solid substances that make up all the objects around us, the transition from a solid to a liquid state and from a liquid to a gaseous state of a substance is also not clear.
It is time to try to rethink the Atomic Structure Model and create a new concept that will be able to explain both the Strength of solids and the elasticity of rarefied Gases.
A new Atom model is proposed for consideration, the main features of which are the following theses:
The nucleus of the atom practically coincides in size with the outer boundary of the atom. The coincidence of the size of the Nucleus with the outer dimensions of the Atom makes it possible to give explanations to many previously unexplained phase states, that is, solid, liquid, and gaseous substances.
Solid substances in the new concept are explained by solid Atomic Nuclei that are in direct contact with each other. Moreover, their tensile strength is determined by the attraction of individual atoms at the level of short-range Magnetic forces in the atomic nucleus. Well, and the strength for all-round compression is determined solely by the strength of the dense atomic nuclei themselves. All-round compressive strength tends to infinity, which is manifested in the very fact of the existence of giant objects strongly compressed by gravity, such as stars and planets.
The case of one-sided pressure does not create conditions of all-round compression, and therefore the atoms begin to slip over each other under the action of shear forces in the perpendicular direction from the acting compression force. It is precisely the possibility of such a shift that is based on all metalworking technologies (pressing, forging, stamping), when in a narrow gap between the rollers it is possible to roll a metal blank into a very thin foil or between the die and punch it is possible to flatten the blank into a “glass” shaped product. That is how thin-walled beer cans are made of aluminum flat cakes with the help of stamping.
The nucleus of an atom has a very clear and unique Structure-Architecture, which is responsible for all the physical and chemical properties of a particular simple substance from the periodic table.
The transition to the consideration of "small" details of the structure of the nucleus allows you to move from the existing negligible Numerical Characteristics of the number of nucleons and the magnitude of the charge on the periodic table to the informative Qualitative Characteristics of the Architecture of the Nucleus Structure, which should carry the entire set of chemical and physical properties of a simple substance.
The transition to different state of aggregation (solid-liquid-gaseous) is carried out by changing the relative position of individual nucleons in the structure of the nucleus or a change in the nutria of the nucleons themselves.
In the model of the Atom of Rutherford-Bohr, the nucleus was thousands of times smaller than the size of the Atom, and therefore the effect of its tiny nucleus on external phase transformations was simply not considered. With a thousand-fold increase in the nucleus size, its role in the influence on all the physical properties of a substance, including phase transitions to various states of aggregation, dramatically increases. With the direct contact of large multi-nucleon Atomic Nuclei, a new type of interaction arises, while being based on the long-known Magnetic and Electrostatic forces.
At close contact of the Nuclei, enlarged to the outer boundaries of the Atom, the previously ignored phenomenon, “Electrostatic and Magnetic Interaction of Short-Range Order”, that is, the interaction of Strong Magnetic and Electrical Charges of nucleons at distances close to the nucleus size and the size of individual nucleons, begins to manifest itself.
The previous model considered the atom electrically neutral, and the separation of the negative charge of the electron shell and the positive nucleus was not taken into account.
In the new model, the interaction of Nuclei-Atoms can be considered at the level of the local effect of the close convergence of like poles of separated intranuclear Charge-Dipoles. Such an approach makes it possible to create a model of solid matter on the forces of magnetic attraction, as well as a model of static gas states on the forces of electrostatic repulsion. The intermediate state in the model of the Liquid phase has elements of the models of the Solid and Gaseous phase, opposed to each other in a narrow gap between the atoms.
Gas exists on the repulsive forces of electrostatic charges of the same name in the dipoles of individual nucleons, when all the electric dipoles of an atom are turned outward by the same electric pole. At the same time, all the neighboring gas atoms are tangled with the same poles of the electric dipoles (the atom remains totally neutral), which creates their local Electrostatic mutual repulsion. (Fig.1, Fig.2, Fig.3).
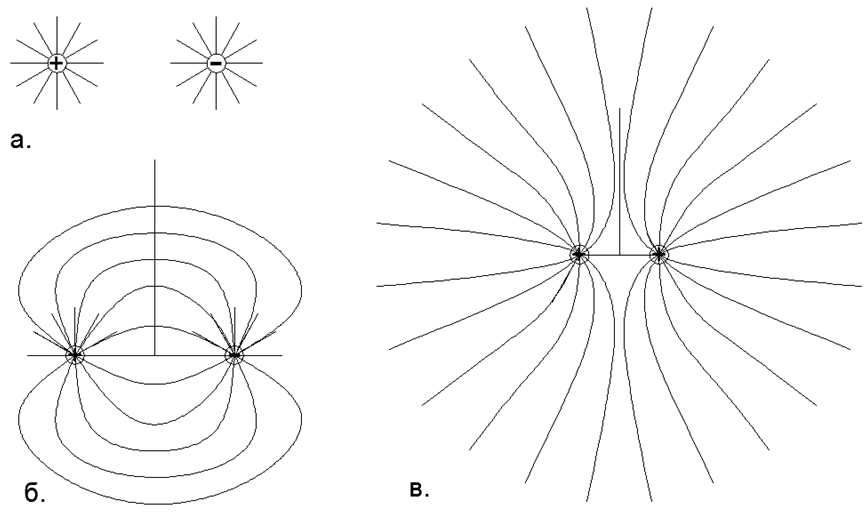
Fig.1. The interaction of fields of point charges according to the principle of superposition: a) Two isolated opposite charges with a correct radially directed (spherical) electric field, where the lines of force are evenly distributed and go along radii to infinity, that is, in an abstract model, their fields do not interact; b) The real shape of the force lines of two closely spaced single opposite charges, where, according to the superposition principle, the positive charge force lines are closed on a negative charge, and at infinity the total charge of this system is perceived as zero and the lines of force do not go from the dipole to infinity; c) The real form of the lines of force of two closely spaced single like and one-dimensional charges, where, according to the principle of superposition, the lines of force of the same charges do not intersect, but are displaced into separate half-spaces, and at infinity the total charge of this system is perceived as a point charge of twice the nominal value.
That is, from a distant macro-environment, neutral atoms appear to be uniformly neutral, whereas in the near-order with neighboring atoms, gases are kept in a consistently equidistant state on the forces of the same electrostatic repulsion.
In the “Electrostatic Short-Range Order” model, gas molecules cease to move with enormous speeds, remaining in a state of electrostatic rest, and their temperature is determined solely by the strength of the electrostatic field in the short-range interaction order. A smooth change in the gas temperature is realized by a smooth synchronous change in the length of the arms of the electric Intercranial Dipoles in the radial direction of the atom. The shorter the dipole arm, the shorter the length of the electrostatic corona petals it can create.
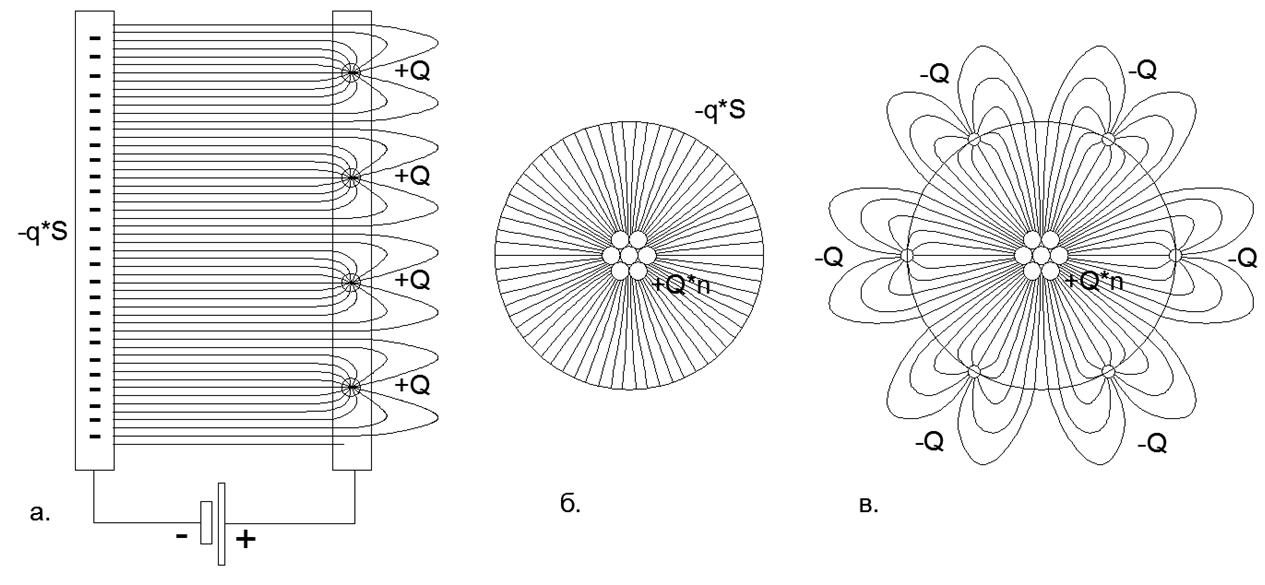
Fig.2 Distribution of the power lines of the electrostatic field on the plates of a capacitor with distributed and point charges: a) Flat capacitor with a distributed charge Negative plates (the field does not intersect the plate) and Single point charges on the Plus plate (the field closes outside the gap between the plates) ; b) Spherical capacitor with a distributed charge on the Minus lining and Single point charges on the Plus lining; c) Spherical capacitor with single point charges on the Plus-lining and Single point charges on the Minus-plate (short-circuiting the field on external charges creates long lobes of lines of force that extend far beyond the solid outer spherical plate of the capacitor).
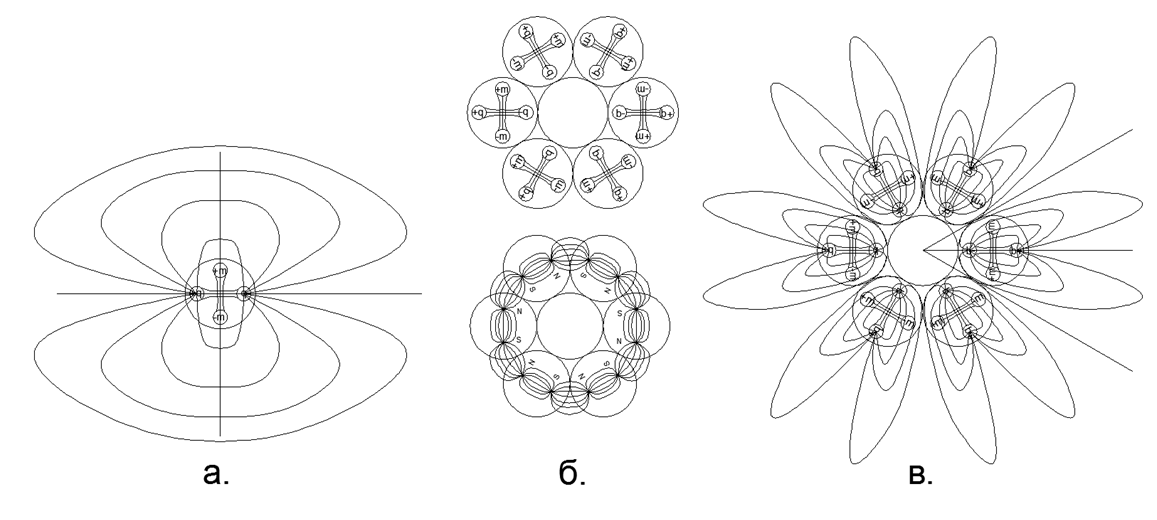
Fig.3. a) View of the electric field of the dipole in the composition of the free nucleon; b) holding on a magnetic force a chain of 6 nucleons closed in a ring, while the magnetic field lines are entirely closed inside the ring and do not go out into outer space; c) The electric field of the dipole of the nucleon ring, which forms the electrostatic Corona (with the petals of the power lines of the electric "crown" going far beyond the limits of the size of the atom).
The electrostatic close interaction of repulsion in gases and the magnetic attraction of nuclei in the liquid (solid) phase well explain the process of vaporization with gradual heating and critical states of vapor on saturation. So evaporation (exit from a solid or liquid phase into a gaseous state) occurs when the atom is heated, the Electrostatic dipoles of the nucleons are turned out by the same poles to the outside to such an extent that at some moment the electrostatic repulsion becomes greater than the magnetic force of neighboring atoms. Condensation occurs in the reverse order: the cooling of the atom leads to a decrease in the strength of the Electrostatic repulsion when the electric dipoles turn to the neutral position.
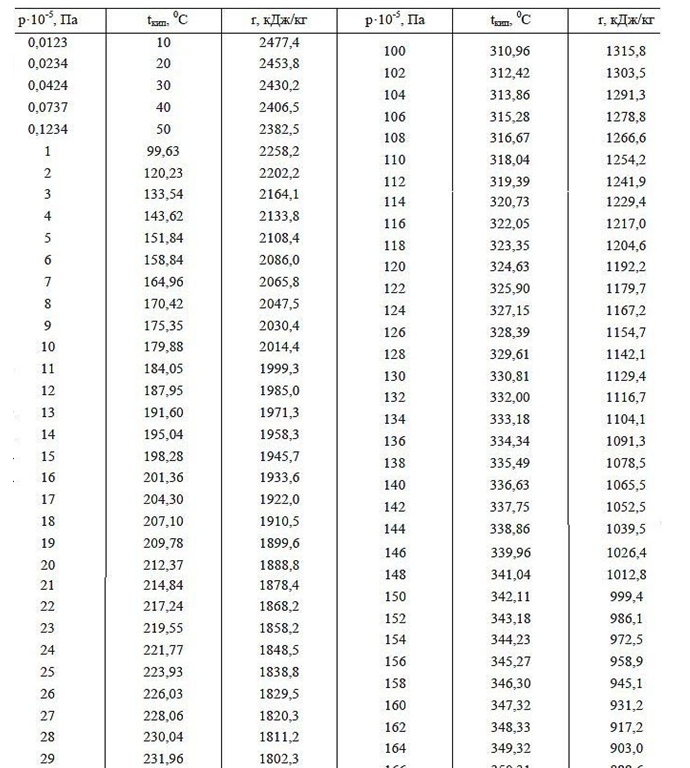
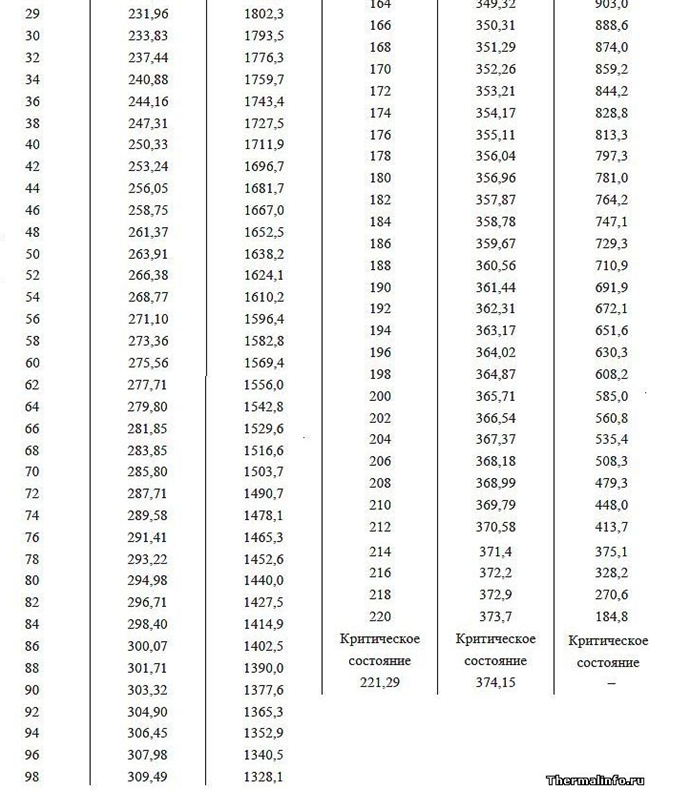
The work function (energy of vaporization) is the additional energy of the field spent by the atom during the expansion of the electrostatic corona in an empty gas space. The output energy (heat of vaporization) with increasing gas pressure should decrease at equal temperature of liquid and gas above it, which is confirmed by the example of the dependence of the value of heat of vaporization at different air pressures around (see tabular data for pressures below 1 atm. In high mountains). Also, when the pressure and boiling point of water increase (tabular data of the boiling point of Water at different pressures above 1 atm.), The work function decreases, which is explained by the reduction in the size of the intermolecular distances in the liquid and gaseous phases, thereby reducing the volume of the Crown electric field, which additionally absorbs energy at its disclosure after leaving the dense water in the rarefied gas phase.
The transition from the state of steam to the state of ice at negative temperatures must pass through the phase of liquid water. At the same time, on the surface of the already solidified ice, a thinnest layer of the liquid phase of water with a temperature above zero Celsius is formed, in which the heat flux from the folding of the field of condensing water molecules is distributed. The greater the temperature difference between ice and 0 ° C, the thinner the layer of water on the surface, since a large temperature difference increases the intensity of heat exchange in the liquid-ice layer, with a proportional decrease in the thickness of the water layer to conduct the desired heat flux from the condensed water molecule.
The thinnest layer of water on the ice surface when interacting with the support from the external load (the sole of the boot or the sledge runners) creates conditions for the appearance of "hydrocline" even at almost zero relative load velocities relative to ice. The thinnest layer of water with a thickness of several molecular rows simply does not have time to be squeezed out of a thin gap, while the viscosity of liquid water turns out to be very low, which creates the possibility of the sole slipping on ice from a water “hydrocline” lubricant.
If the thickness of hydroclin is related to the temperature difference with respect to 0 ° C, then the ice sliveriness should decrease with increasing frosts. It is this behavior of the Icebreaker that can be traced in practice, when the greatest slip on the roads is noticeable at temperatures around zero Celsius. In severe frosts, when driving on ice, direct contact is solid-solid with almost no formation of hydrocline, as if instantaneous molecular seizure of the ice with the sole (tire) occurs, freezing the ice to the ice.
According to some data, the thickness of the water film on the surface of ice, which is equal at -5 degrees to 100 nm, at -35 degrees decreases tenfold to 10 nm, and at -170 degrees it consists generally of a single layer of molecules. So, the inhabitants of the Arctic say that dragging sleigh on ice at very low temperatures is the same as dragging them across the sand (there is little lubrication in this case).
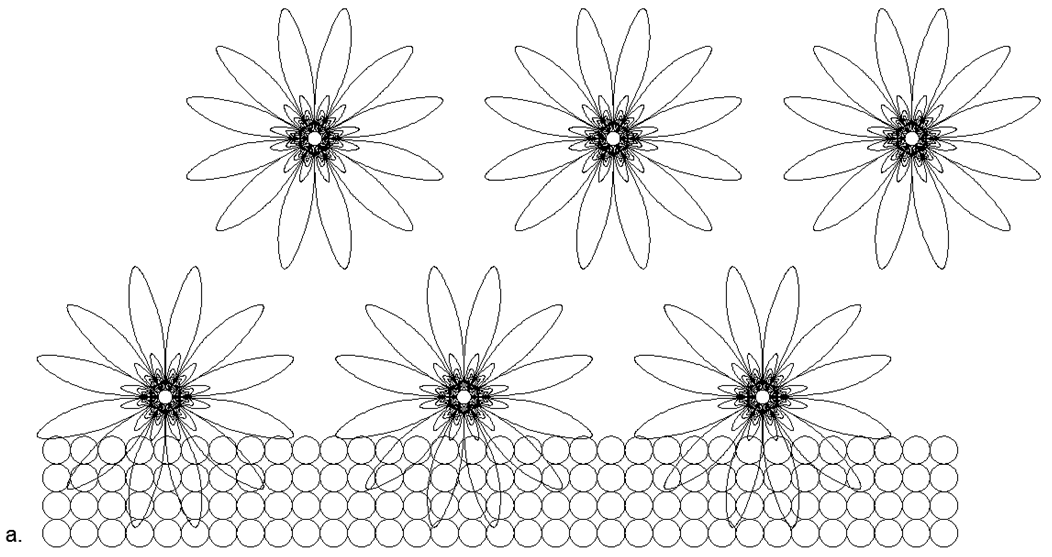
Fig.4 Schematic representation of the boundary zone of contact of different phases Liquid-Gas (Water-Air) in the case of Electro-Static Theory of the existence of Gaza: a) Gas at a pressure of 1 atm. above liquid water (the distance between the centers of the molecules is approximately 10 times greater than in the liquid phase); b) The same gas above water at the same temperature, but at a pressure of 10 atm., while the distance between the centers of the gas molecules is reduced by 10 ^ 1/3 = 2.15 times. One can clearly see the deformation of the contacting monopolar electric corona of gas atoms when molecules approach each other. The lines of force can not intersect, and therefore are forced to deform, occupying a smaller volume, which leads to an increase in mutual repulsion forces (increase in gas pressure).
Tab.1. Heat of vaporization for water depending on pressure and temperature.
The existing equations of state of an ideal gas (Boyle-Marriott law) well describe gases at low pressures (normal conditions) and high temperatures. But at high pressures, this ideal law does not allow one to describe transitions to either a liquid or, moreover, to a solid state.
The van der Waals law tries to correct inconsistencies by introducing additional factors, fitting the theoretical curve to the experimentally derived relationship. In this case, there is a hidden rejection of the Rutherford-Bohr atom model, since in the van der Waltz equation, the atom is represented by a solid ball that fills the entire volume at its outer boundaries.
In this case, the nature of the interaction of the atoms of the balls is not qualitatively explained, leaving explanations at the level of weightless statistical electron shells over the tiny nucleolus, as it should be according to the Rutherford-Bohr model.
In the case of the proposed model, where repulsion of like-charged surfaces occurs, it becomes possible to consider an Understandable Static Model, where each element of the model has a clear physical embodiment and physical meaning.
So the interaction of dipoles is expressed by the simplest formula of direct pairwise interaction for four point charges assembled in pairs in mechanically strong dipoles with an arm L and the distance R between the proximal ends of the dipoles:
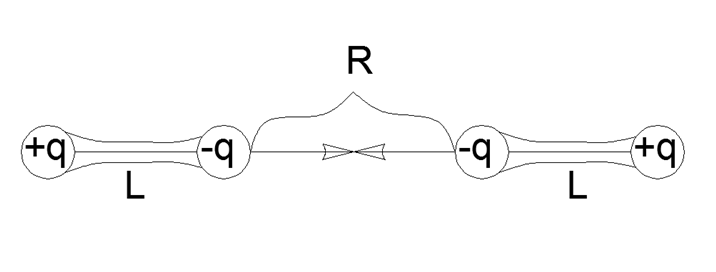
F = K q2 (1 / R2 - 2 / (R + L) 2 + 1 / (R + 2L) 2)
Having led to a common denominator and opening the brackets in the numerator, we get an even more cumbersome expression:
F = K q2 (2R2L2 + 12RL3 + 4L4) / (R2 (R + L) 2 (R + 2L) 2)
A feature of the computational model for dipoles is that the repulsion function of the dipoles rushes to Infinity away from the center of the atom, or rather at its outer edge, where direct contact of atoms is possible when exposed to the most powerful forces of magnetic atomic attraction. It is this kind of contact between two forces that vary sharply in magnitude and opposite in sign on the atomic border and allows us to create a model of balanced Repel-Attraction of atoms with each other.
If R >> L, the function of electrostatic repulsion of dipoles is simplified to the form:
F = K q2 2L2 / R4
Magnetic attraction has a saturation property and the graph of the function does not go to infinity even with direct contact of atoms, so Magnetic attraction will always find an equilibrium point with Electrostatic repulsion on the steep hyperbolic slope of the Electrostatic characteristic.
Of course it is clear that in reality there are no infinitely large forces, but there is a certain limit of maximum values (for example, the attractive forces of atoms can be estimated by direct measurement of the tensile strength in a single crystal of a substance, and the finiteness of the electrostatic interaction forces is determined by the fixed energy during the electron annihilation with Positron). When two atoms approach each other, the Repulsive Forces at some point also cease to grow rapidly and reach a certain fixed final value. Thus, two not infinitely large values of attraction and repulsion balance in a certain point of mutual equilibrium, which can be found at the intersection of the graphs of these two functions, when plotting graphs in one coordinate system. (see Graph 1)
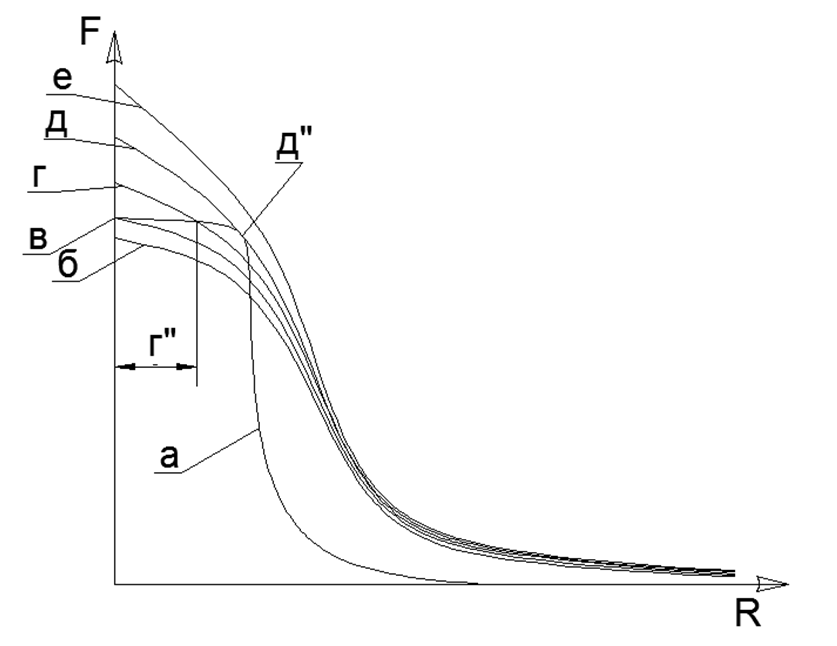
Graph 1. Graphs of the active forces of magnetic interatomic attraction (a) and electrostatic repulsion forces at different temperatures: in solid matter (b); at melting T (c), with molten liquid state (g) with an interatomic gap (g "); critical vapor-liquid equilibrium temperature (e) with a touch of repulsion curves and attraction at point (d "); superheated steam (e). The R axis is the distance between the surfaces of the outer boundaries of the nearest contacting atoms. At the same time, the area of the closed segment between the curves (r-a) is equal to the output energy (detachment) of the atom from the liquid phase array, and the region to the right of the intersection of the repulsion curves with attraction is the gas phase region, where the area under the repulsion curve to the attraction curve is equal to the field work energy during vaporization (field expansion after separation from liquid) at given temperature (one of the letter curves) and gas pressure (R max is the distance between gas molecules at a given pressure).
The van der Waals equation draws a very similar picture, but does not explain the physical meaning of the forces of attraction and repulsion, while slyly pushing the zone of infinite asymptotic growth of the function into the inaccessible zone of the center of the atom.
The liquid state of a substance is the moment when the heating of the Electrostatic dipoles to the repulsion has already begun when heated, but this repulsion is not yet able to break the bond of the magnetic attraction of the atoms. As a result, a small gap appears between the atoms (g ”in Graph 1), leading to a sharp increase in the mutual mobility of the atoms on the layer of electromagnetic“ lubricant ”.
The solid-gas boundary layer is formed when a magnetically coupled solid substance comes into contact with electrostatically stressed gas atoms. Gas atoms cannot repel themselves from a solid substance with unopened electrostatic coronas of atoms and are pressed to a solid surface. This forms the boundary layer of Gaza atoms pressed to the surface of a solid substance.At the same time, the boundary gas layer is still capable of pushing away from itself other gas atoms, since the boundary gas layer remains electrostatically stressed to the necessary degree. Such pressing of the boundary layer of gas (air) into a solid metal explains the almost instantaneous appearance of metal oxides on the surface. Further contact of the boundary layer of the gas is already carried out with a solid protective film of oxides on the metal surface, which protects the underlying layer of pure metal from further oxidation.
Modeling alleged Structures-Architectures of chemical elements. In the future, the proposed models of the structure of the nuclei of atoms of various substances are considered, where the metal ball with strong magnetic properties will act as a nucleon (neutron, proton). The magnetic interaction of nucleons meets the criterion of the Non-centrality of the force interaction of nucleons in the nucleus and the saturation of these forces when nucleons approach each other, which has long been assumed in Nuclear Physics for strong nuclear interactions.
Modern rare-earth magnets have a very high magnetic force, which makes it possible to build plausible models of atomic structures, relying only on the magnetic holding forces of the Sharik-Nucleons as part of a single core with a unique Architecture. At the same time, the magnetic forces themselves act as Critics Controllers for each such model of nuclei, since not all spatial combinations of balls can be assembled in a stable form due to the mutual attraction-repulsion of polarly magnetized balls.
The model of each atom is assembled from the number of nucleons given by the periodic table (including all stable isotopes). Reconstructions of models are created from neodymium magnetic balls with a diameter of 5 mm. Currently, these magnetic balls are sold as puzzle toys called Neokub, which includes 216 magnetic balls of the same size (cube 6x6x6 pcs.).
Based on the obtained geometric features of the atomic models of each simple substance, we will try to find logical connections between the shape of the atomic model and the properties of the real substance at the macro level.
At the initial stage of the simulation of nuclear structures, some regularities emerged. So the magnetic balls tend to line up in chains, and quite long chains easily close into rings. Ring magnetic structures are very strong and stable, while the external magnetic field of these rings is sharply reduced, since almost all the magnetic lines of their magnetic flux separate magnetic balls are closed through adjacent balls of their magnetic nucleon ring (see Figure 5). Rings of the same size (an equal number of nucleon balls) are able to easily connect with each other, forming very stable columnar structures that can grow almost infinitely along the length with new commensurate rings (Fig.6).
The chains of nucleon balls can be connected in parallel to each other in two versions:
The passing direction of the magnetic fluxes gives a more solid and compact connection of chains of balls (the balls are located at the vertices of isosceles triangles). Counter-directed magnetic fluxes cause the balls to be located on the tops of the squares, which gives a less dense and less durable packaging of nucleon balls
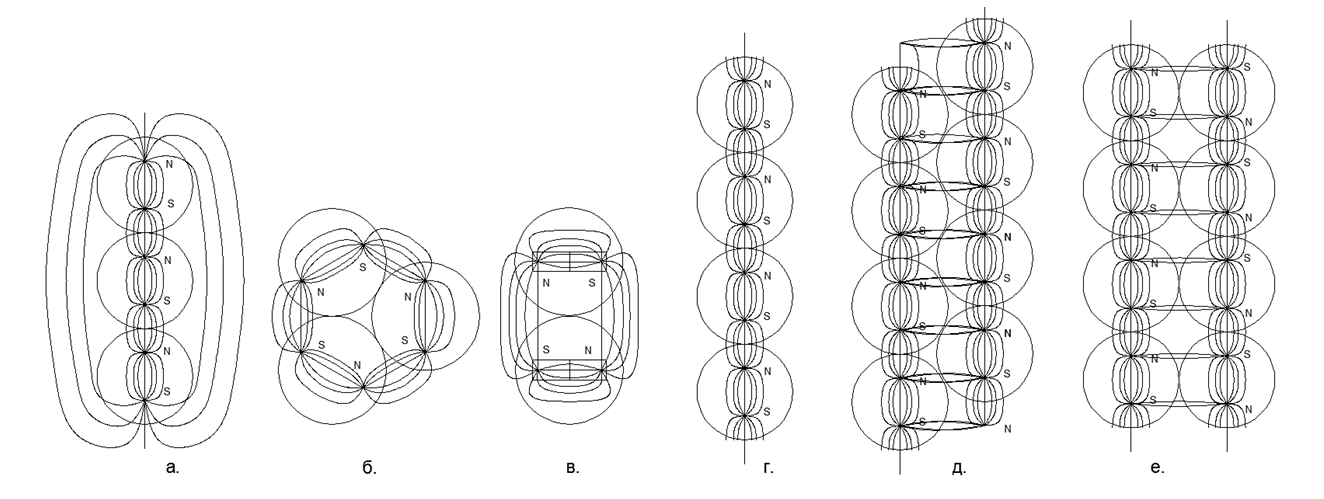
Fig.5. Options for the interaction of magnetic balls in the simplest versions:
but. Linear column of 3 magnetic balls (steady);
b. An annular closure of the magnetic flux itself on itself from 3 balls (steady);
at. Кольцевое замыкание магнитного потока самого на себя из 2-х шаров (неустойчивое), при малейшем сдвиге перестраивается в столбовой вариант по типу (а.);
г. Развёртка замкнутого в кольцо магнитного потока одиночной цепочки шаров- нуклонов;
д. Развёртка замкнутого в кольцо магнитного потока сдвоенной цепочки шаров- нуклонов с попутным расположением магнитных потоков, при этом шары расположены по вершинам равносторонних треугольников, что обеспечивает максимально плотную и жёсткую к сдвигу пространственную упаковку;
е. Развёртка замкнутого в кольцо магнитного потока сдвоенной цепочки шаров- нуклонов со встречным расположение магнитных потоков, при этом шары расположены по вершинам квадрата, что обеспечивает не самую плотную и нежёсткую к сдвигу пространственную упаковку. При встречных магнитных потоках в случае сдвига цепочек связи между рядами резко слабеют.
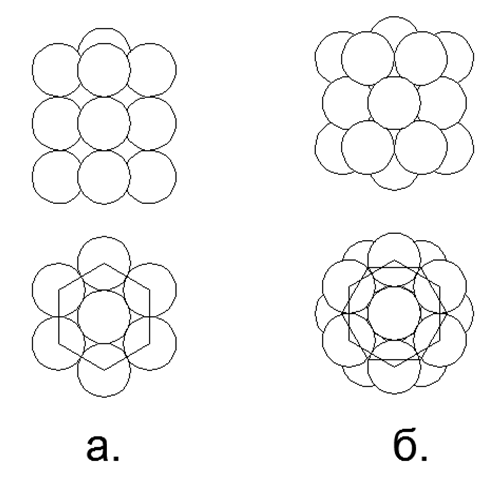
Рис.6. Магнитостабильные Столбы из 6-нуклонных магнитных колец:
but. Трёхрядный столб с противоположно направленными магнитными потоками в кольцах;
b. Трёхрядный столб с попутно направленными магнитными потоками в кольцах.
Попутное соединение магнитных потоков с круглыми магнитами-шариками оказывается более прочным. На видах сверху в центре 6-ти нуклонных магнитных колец виден просвет, куда свободно помещается по одному шарику в каждом кольцевом ряде.
.
In addition to combining one-dimensional rings, it is possible to join and different-sized rings in various combinations, giving a variety of spatial forms. It is precisely this variety of possible forms of combining ring magnetic structures and individual magnetic balls that makes it possible to create many models of the Architecture of nuclei with a similar number of nucleons, but related to different substances with radically different chemical and physical properties.
As is known, a set of isotopes with different numbers of neutrons in a composition can correspond to a single chemical element. Thus, the Pillar structures of atoms from rings with six or more nucleons have a lumen in the center, which allows placing additional nucleons inside the column without changing the appearance of the atomic nucleus. (Fig.6.b).
Based on the proposed version of magnetic attraction and electrostatic repulsion, it can be assumed that metals include nuclear structures that have an additional outer ring of magnetically coupled nucleons on top of a central column of one-dimensional nucleon rings (Fig. 7). This assumption is based on the fact that the external magnetic ring is not charged, thereby increasing the gap between the internal electro-charged column when approaching other atoms of the same type. Such a gap raises the boiling point and melting point of the metal, as well as creates a wider area for the liquid state zone.
Substances that are Gases under normal conditions, on the contrary, should have a charged nucleon ring in the outer loop, providing electrostatic repulsion from gas atoms of the same type, while a large number of nucleons can be placed inside the charged ring, creating a wide isotope series of this element.
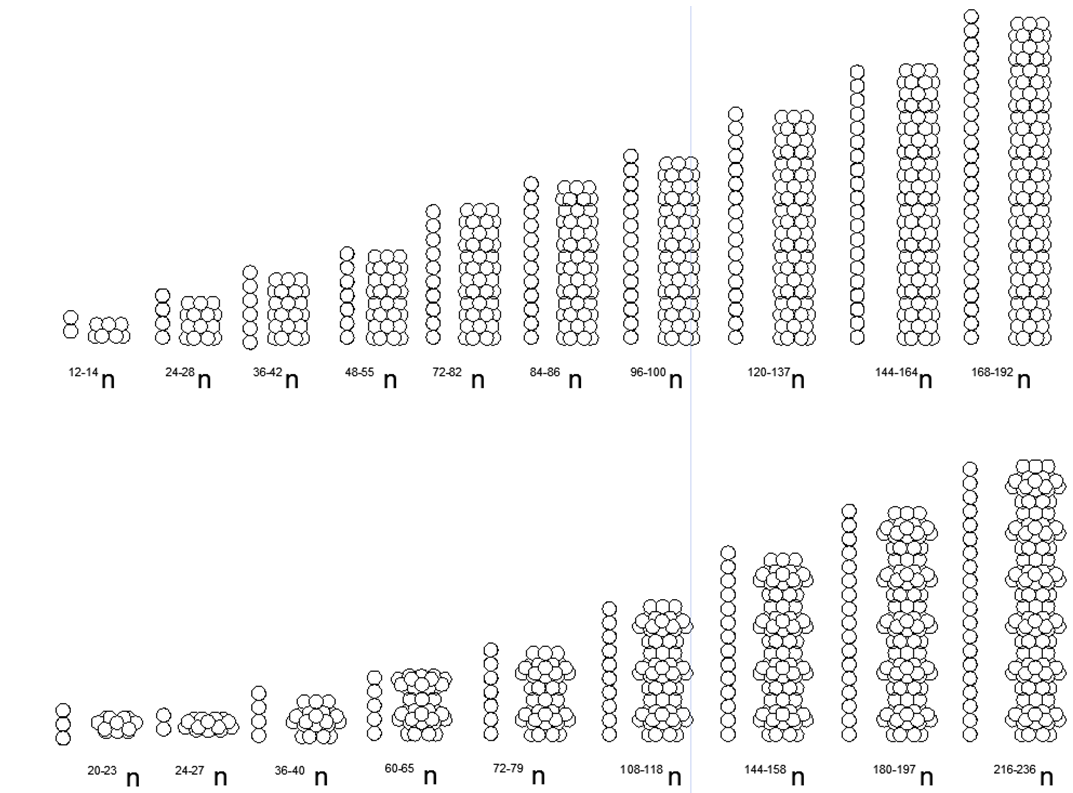
Fig.7. Возможные Магнито-Стабильные Варианты соединения столбовых структур с одноразмерными ( верхний ряд — неметаллы) и разноразмерными ( нижний ряд- металлы) кольцами и с различными вариантами заполнения центральных просветов для различных вариантов нуклонного состава. Рядом с рисунками основной кольцевой конструкции указано количество шариков, свободно размещаемых внутри просвета магнитных колец, с получением диапазона изотопов к данной основной конфигурации. На рисунке показаны далеко не все возможные конфигурации атомных магнитосцепленных структур, так как разнообразие всевозможных комбинаций разнонуклонных колец крайне велико, что не позволяет их все показать на одном маленьком изображении.
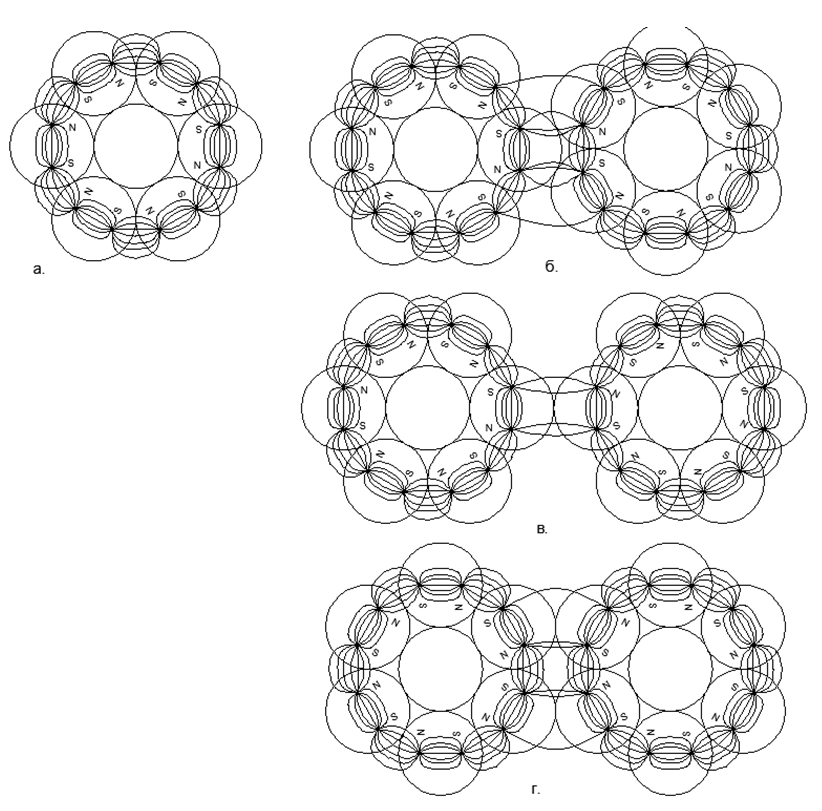
Рис.8. Взаимодействие магнитных колец при «точечном» контакте атомов с образованием межатомной связи магнитного притяжения:
but. Магнитный поток в 6-ти нуклонном кольце в свободном состоянии;
b. Искажение магнитных линий в 6-ти нуклонных кольцах в месте внешнего касания с образованием химической связи на притяжение при попутном магнитном потоке колец в точке касания, контакт максимально жёсткий и плотный с касанием в двух точках, такая химическая связь наиболее прочная;
at. Искажение магнитных линий в 6-ти нуклонных кольцах в месте внешнего взаимного касания с образованием химической связи на притяжение при встречном магнитном потоке колец в точке касания. Контакт нуклонов происходит в одной точке, а потому связь подвижнее и слабее, чем при попутном контакте, при этом связь стремится трансформирватся в двухточечную устойчиву (рис.г).
г. Искажение магнитных линий в 6-ти нуклонных кольцах в месте внешнего взаимного двухточечного касания при встречных магнитных потоках. Контакт нуклонных колец происходит в двух точках, а потому связь достаточно прочная.
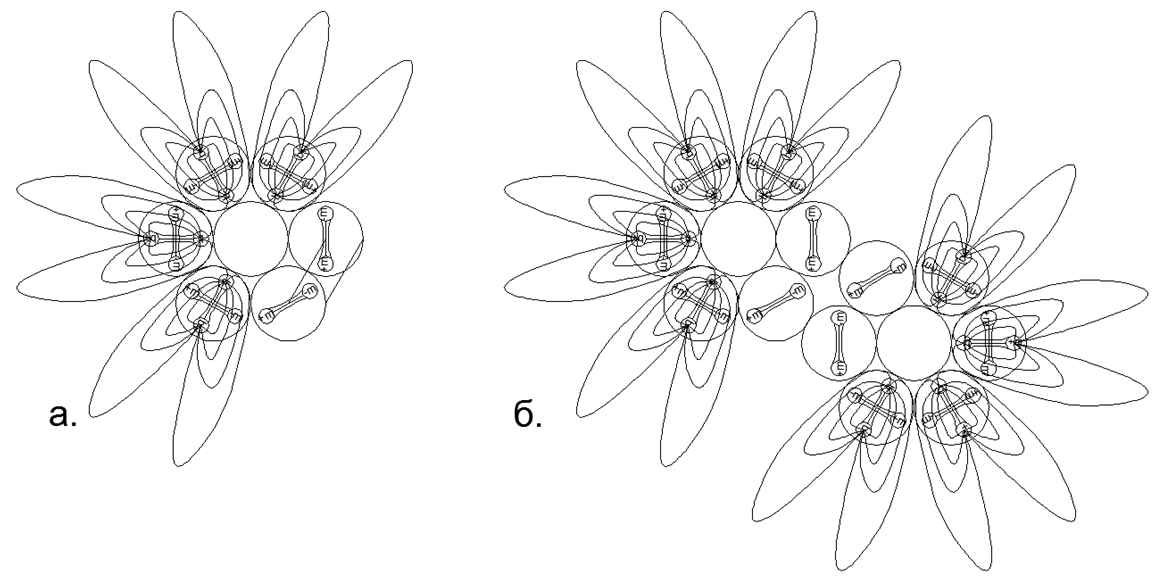
Рис.9. а) Вид неполной электростатической Короны одиночного атома вещества, которое встречается в газообразном виде только как Двухатомный газ ( N2, О2 и т.д.),; б) вид полной электростатической Короны двухатомного газа, при заполнении дыры в электрической короне одного атома неполной Короной другого такого же атома вещества.
In the following, we will look in detail at the most likely Structures - the Atomic Architecture of individual elements with reference to the features of their nucleon composition and known physical properties (density, strength, Tflav, Tcip, etc.).
The following table shows the 1-2-3 periods (short) of the periodic table, where instead of the fractional atomic weight indicated are the integer weights of individual stable isotopes found in nature (Table 2).
Tab.2. Atomic weights of stable isotopes of chemical elements of the 1-2-3rd periods of the periodic table.
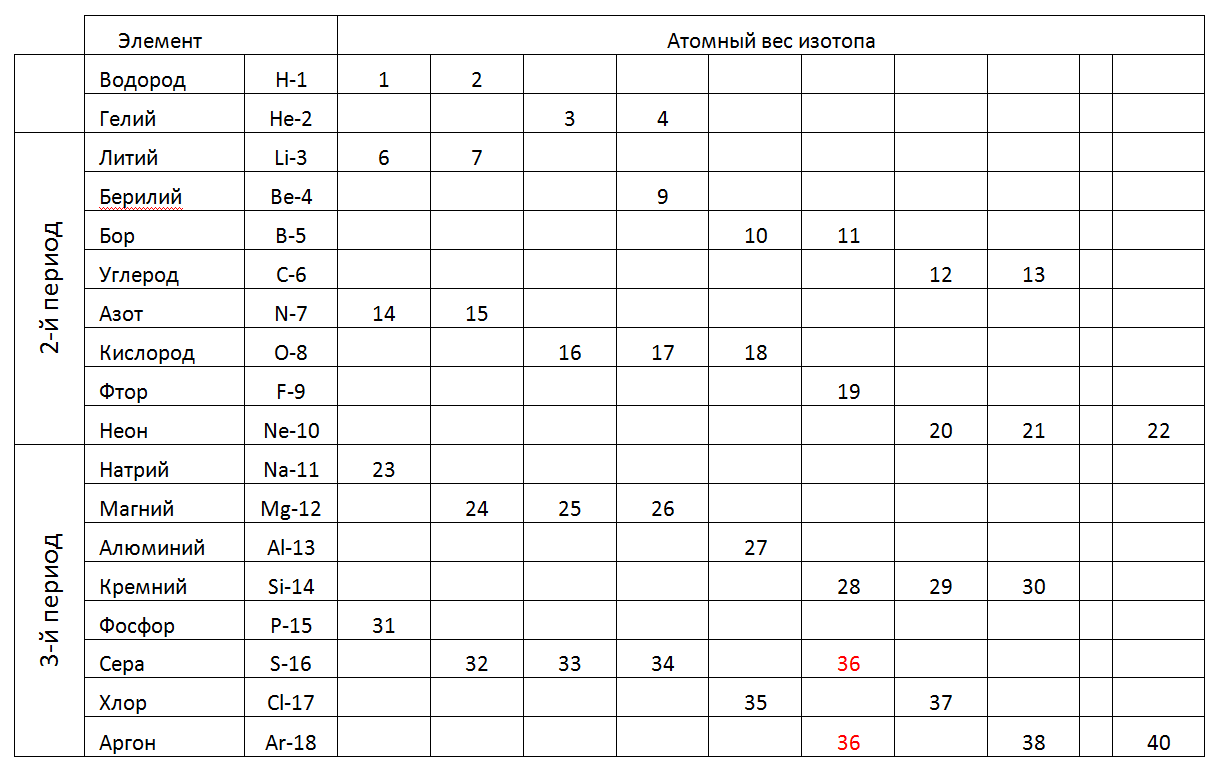
In the 1st period and the 2nd period (the first full row of the table) of the periodic table contains elements with a small number of nucleons. Small-sized atoms of these periods allow us to consider such spatial models, which correspond to unique forms that can set the direction for constructing models of atoms in all subsequent periods (See Figure 7). Moreover, the most reasonable variant of modeling can be considered a gradual increase in the length of the main charged ring of the Atom, complementing it to the maximum nucleon composition with external (metal) and intra-ring nucleons (non-metal) in accordance with their chemical status, and then looking for an analogue according to the table of nucleon correspondences of chemical elements .
1. Hydrogen (11H) 1proton (1p). The free substance has the form H2.
The construction of the hydrogen atom does not require any effort, since it consists simply of a single proton ball. Because of the open magnetic flux, monoatomic hydrogen is extremely active, which causes it to react with the nearest free substances or contact other Hydrogen atoms to a stable closed ring state.
Deuterium (21D) 1 proton + 1 neutron (1p + 1n) - the form is also unique in the form of two stuck balls.
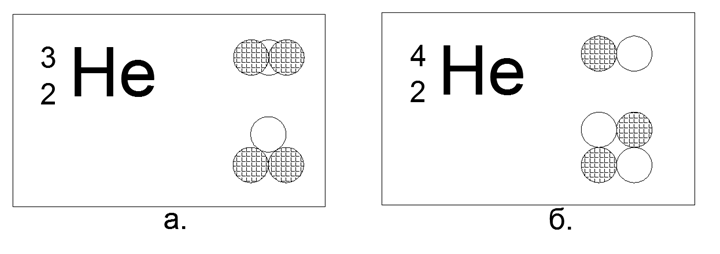
2. Helium (42He) 2 protons + 2 neutrons (2p + 2n)
For Helium, it is already possible to build several variants of the nuclear structure models:
In this case, of the 4 magnetic balls, it is possible to build only the flat Square shape of the nucleus. The tetrahedral form on the magnetic forces is not held, turning immediately into a square.
In the future, we will focus only on the spatial forms of atoms, since linear variants in more massive atoms cannot give any meaningful incarnation.
Rare isotope Helium (32He) 2 protons + 1 neutron (2p + n)
In this configuration, only a flat triangle is possible.
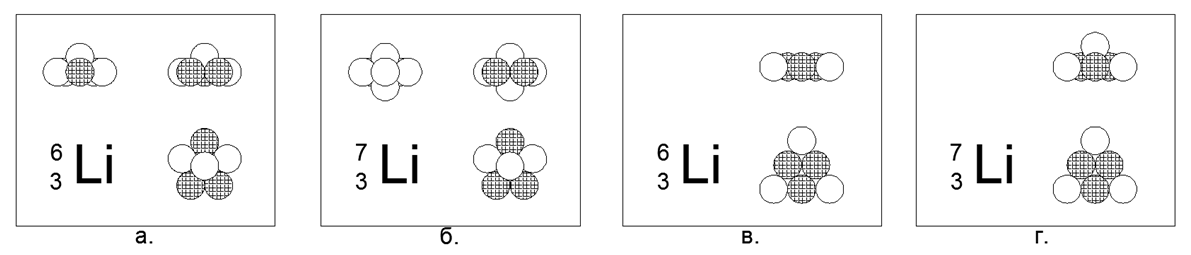
3. Lithium (73Li) -7 nucleons (3p + 4n)
For Lithium, it is possible to create both a flat and a bulk model of an atom.
Plane model is a hexagon with one nucleon in the center. A volumetric model is a flat pentagon with two balls at the poles (Poles are points at the ends of the axis of rotation of the main axisymmetric part of the atomic nucleus). Both models have a pronounced star-like symmetry. Lithium is a metal, very light (0.534 g / cc), relatively fusible (Mp = 454K). The rare stable isotope Lithium (63Li) -6 nucleons (3p + 3n) can be modeled in both forms, so the choice in favor of any model cannot be made.
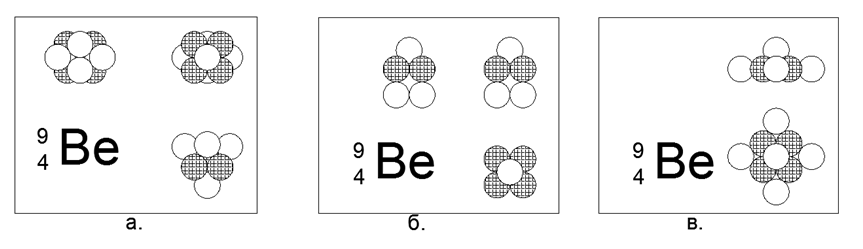
4. Beryllium (94Be) - 9 nucleons (4p + 5n).
The only stable long-lived isotope. There is a relatively long-lived isotope Beryllium (104Be) - 10 nucleons (4p + 6n) with a half-life of 1.4 billion years. Many implementations are possible. The most stable and compact form of sensations looks like a star-shaped form of two triangles connected by planes to a prism, where one side of the triangular prism is attached to the side faces. Beryllium is a light metal (1,848 g / cc), relatively refractory (Tpl = 1551K). In the future, the STARNESS will be traced in most metals. When the 10th ball is added to the resulting structure, the form itself is modified into another star shape, but asymmetrical in the plane. A new form can be described as a six-pointed star with a filled center, with a triangle of nucleon balls attached to its side plane.When viewed along the axis of the "star" at both forms of Beryllium isotopes, a single-type three-pointed star is clearly visible, determining the basic external properties of a simple substance.
5. Boron (115Be) - 11 nucleons (5p + 6n).
Stable long-lived isotopes 10B and 11B. The spatial configuration of 10V consists of two pentagonal rings connected parallel in the passing or opposite direction of the magnetic flux. The spatial configuration 11B repeats the structure 10B, only one neutron is added along the axis of the rings.
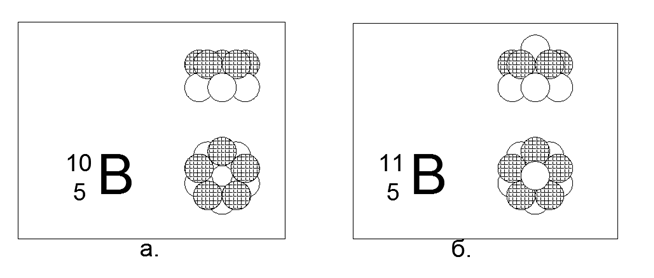
Thus, it can be assumed that a non-metal differs from a metal in its basic external shape: a non-metal is a cylinder of similar rings, a metal is some star-shaped (disk-shaped) shape.
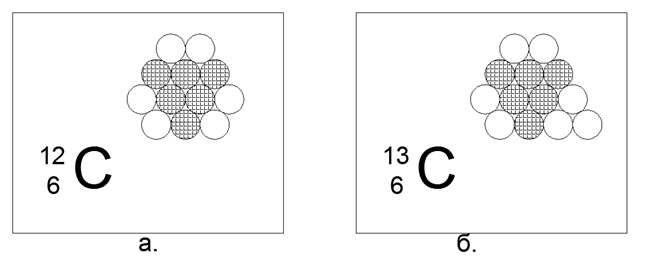
6. Carbon (126C) - 12 nucleons (6p + 6n).
Stable long-lived isotopes 12C (98.93%) and 13C (1.07%), as well as radioactive 14C. An extremely interesting element that is very common and has an infinite variety of structural forms in nature (Graphite, diamond, coal, soot, nanostructures-fullerenes, etc.) This form-content requires some amazing special properties from the structure of 12 balls. Such a structure can be a flat, non-equilibrium hexagon with three balls in the center.
The charged nucleons are arranged in a triangle, on the sides of which three pairs of neutrons are attached. This results in three-beam symmetry, where external neutrons are located on the symmetry axes, ready for the magnetic coupling of other atoms. Of such hexagons, Layers of Graphite, or the surface of nanotubes and three-dimensional structures of closed Fuleren structures, are easily laid out.

Fig. View of a layer of graphene (graphite) composed of hexagonal flat C12 carbon atoms.
In the series of non-metals, those substances that, under normal conditions, are gases, stand out. These are monatomic Inert (noble) gases, atmospheric diatomic Nitrogen and Oxygen, and active diatomic halogen Fluorine.
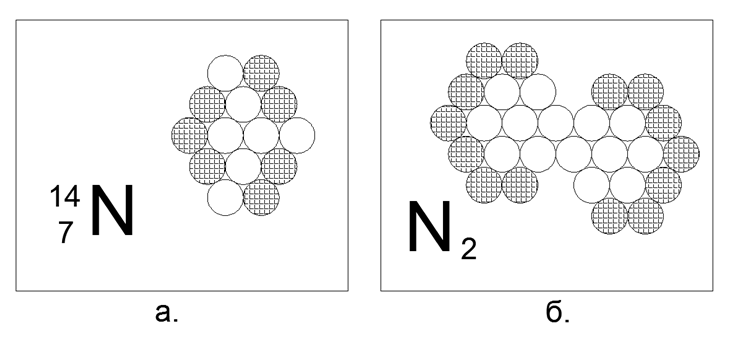
7. Nitrogen (147N) - 14 nucleons (7p + 7n).
Stable long-lived isotopes 14N and 15N. In its free state under normal conditions, Nitrogen is a diatomic gas, and it is very little active. 78% of nitrogen is the atmosphere of our planet. To possess the property of gas at N.u. the atom is required to have charged nucleons on the outer perimeter, without discontinuities for pair neutrons. But since the gas is only a diatomic molecule, it means that in the structure of the atom there is a magnetoactive segment of two or three neutrons in a row on the outer border, which are the atoms of the Nitrogen in the N2 molecule. Such a form of 14 atoms is easily assembled in the form of a nonequilibrium hexagon, similar to a rhombus, where you really eat a site with three neutrons in a row on the outer perimeter of the atom. The bond in the N2 molecule is so strong that the gas remains in a molecular state even at 5000 degrees C.
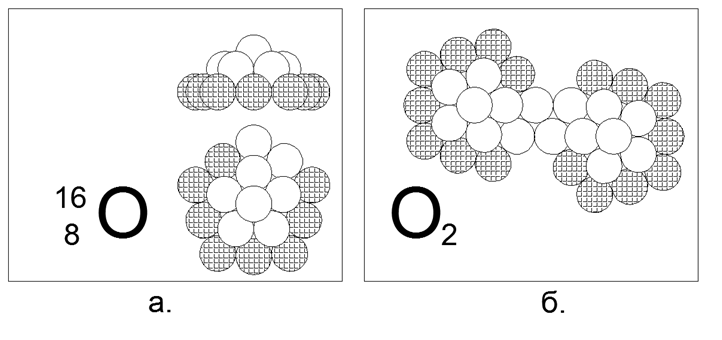
8. Oxygen (168O) - 16 nucleons (8p + 8n).
Stable long-lived isotopes 17О and 18О. The estimated form of the most common (99.7%) isotope 16O is a 5-square of ten nucleons (8 charged, 2 neutral), with a pronounced magnetic neutral-magnetic contact space in the outer ten-atom ring. The inner liner consists of a 5 nucleon ring with an additional sixteenth nucleon in the center. 10 and 5 nucleon rings can not be joined in a plane, and therefore create a spatial dome-shaped form. Isotopes are created by the capture of additional neutrons in the center under the "dome". The presence of an external two-nucleon gap in the ring ensures the creation of a diatomic O2 molecule. The bond strength inside the O2 molecule is much weaker than that of Nitrogen, and therefore oxygen is much more active and enters into oxidation reactions (burning) at sufficiently low temperatures.
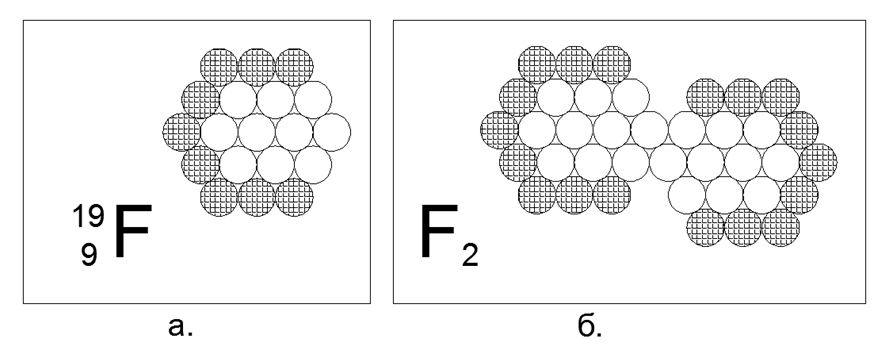
9. Fluorine (199F) - 19 nucleons (9p + 10n).
Only one stable isotope, unstable live from fractions of seconds to units of hours. The assumed form of the structure: a flat hexagon of nested 12 and 6 nucleon rings and the 19th nucleon in the center. The lack of charged nucleons in the outer ring creates a magnetic attraction region of three neutral nucleons, allowing the creation of a diatomic F2 molecule, after which the substance exhibits the properties of a gas under normal conditions. Fluorine-2 is a cryogenic gas, that is, it becomes liquid only at extremely low temperatures (85K or -188C), which makes it related to the properties of the two previous diatomic gases.
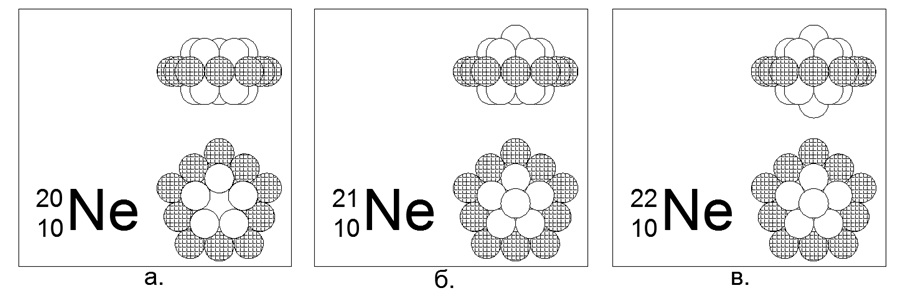
10. Neon (2010He) - 20 nucleons (10p + 10n) content in nature of 90.47%.
Stable long-lived isotopes 21He (9.25%) and 22He (0.27%). Estimated nucleus structure: 10-nucleon charged outer ring plus two inner nested 5-nucleon rings.
Isotopes 21 and 22 are created by adding one nucleon to the center of one of the 5 nucleon rings along the axis.
In Table 1, substances with a single stable isotope are of the greatest interest, since their Unique structure should not allow one to accept additional nucleon balls without introducing distortions into the external form. Such substances include Metals Beryllium-9 (Figure 7) and Sodium-23 (Figure 6), Aluminum-27, as well as non-metal Fluor-19, Phosphorus-31.
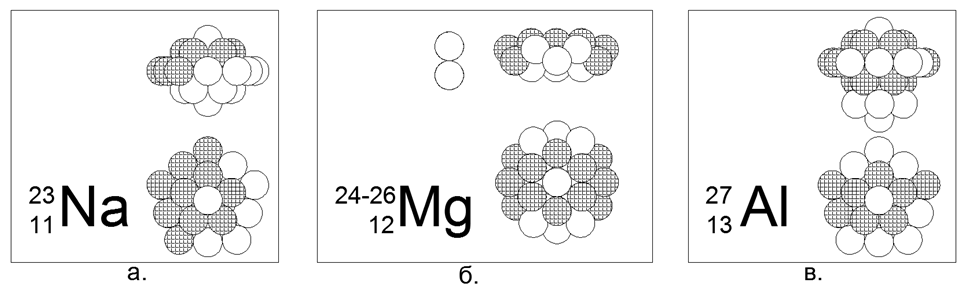
11. Sodium (2311Na) - 23 nucleons (11p + 12n) - This is the only stable isotope in nature, which allows you to choose a unique nucleon configuration for it.
Of the 23 balls, only one version of the dense, symmetric magnetically stable star-disk structure was created. A ring of 10 nucleons is wound around the central part of two laminated planes of 5-nucleon rings. Inside the central part between two 5-nucleon rings, the central 21st nucleon is driven in. From the central part along the central axis one more nucleon is attached (the 22nd and 23rd). In general, the core looks like a yula in the form of a convex lentil. Due to the fact that 5-nucleon and 10-nucleon rings can not line up in a plane, the shape of a 10-nucleon ring acquires a zigzag shape, while the inner 5-nucleon rings are somewhat apart, which allows 21- to fit in the center between them. nucleon.
12. Magnesium (12Mg) - 24, 25, 26 nucleons.
Isotopes have a prevalence of 78.6% - 10.1% -11.3%, respectively. Thus, it is obvious that the main form is a disc-shaped structure of two 6-nucleon rings in the central part and a 12-nucleon ring, wound around. Isotopes are created by driving one or two neutrons into the central tube of a disk, which in a 6-nucleon ring exactly corresponds to the size of a ball-nucleon.
13. Aluminum (2713Al) - 27 nucleons (13p + 14n) - This is the only stable isotope in nature, which allows you to choose a unique nucleon configuration for it.
Of the 27 balls, we managed to create an axisymmetric version of a dense magnetically stable star-like structure with a multiplicity of rings of 5 nucleons: a central column of three 5 nucleon rings and an outer 10 nucleon ring, and two nucleons at the ends of the central tube complete the picture. Isotopes 28 and 29 have a half-life of 2 minutes and 6 minutes, respectively. Isotope 26 - has T1 / 2 = 717 thousand years., And naturally decays into stable Magnesium-26 by electron capture (beta capture). The continuous synthesis of Al-26 takes place in the atmosphere when cosmic fast Protons collide with argon atoms.

14. Silicon (14Si) - 28, 29, 30 nucleons.
Isotopes have a prevalence of 92.2% - 4.7% -3.1%, respectively. Silicon is a non-metal. Under normal conditions, silicon exists in various forms, and all of them are solid substances. In crystalline form, silicon is a semiconductor, which makes it related to carbon from the previous period.
For 28 nucleons, there were no regular forms from a cylindrical row, which made it possible to search for a possible model of an atom in a series of polyhedral plates, such as Carbon and Nitrogen. So 28 nucleons are obtained from two planar hexahedral nitrogen atoms connected by planes.
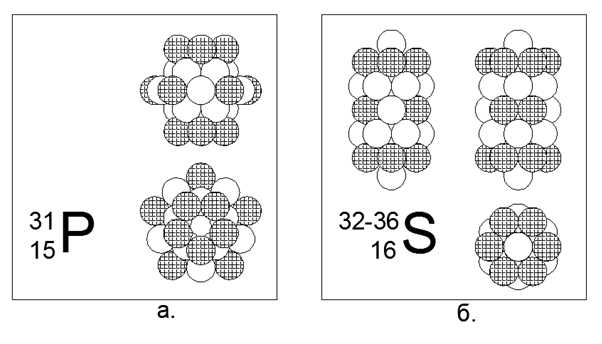
15. Phosphorus (3115P) - 31 nucleons.
The only stable isotope. Non-metal. Estimated structure: Four 5-nucleon rings in the central tube and a 10-nucleon outer ring in the middle of the central tube, which gives 30 nucleons, and 31 nucleons are hammered into the center of a 10-nucleon ring, where a cavity arises due to non-flat joining 10 -ti and 5 nucleon rings. The charges are arranged in 5 pieces at the ends of the central tube and five more evenly around a 10-nucleon outer ring.
16. Sulfur (16S) - has four stable isotopes of 32, 33, 34 and 36 nucleons.
Isotopes have a prevalence of 95.013% - 0.75% -4.215% -0.017%, respectively. Sulfur is non-metal. We assume that the main form is a cylindrical structure of five pieces of 6 nucleon rings, in the central tunnel of which additional isotopic nucleons are placed. For the rare Sulfur-36 isotope, the structure becomes poorly understood, since two additional nucleons hardly fit inside the central tube. Sulfur-36 not only completely fills the inner tube of the cylinder, but also sticks out with nucleons beyond the boundaries of the cylindrical outer part. This configuration gives the typical non-metallic properties of the substance, that is, with relatively moderate melting and boiling points close to normal conditions.
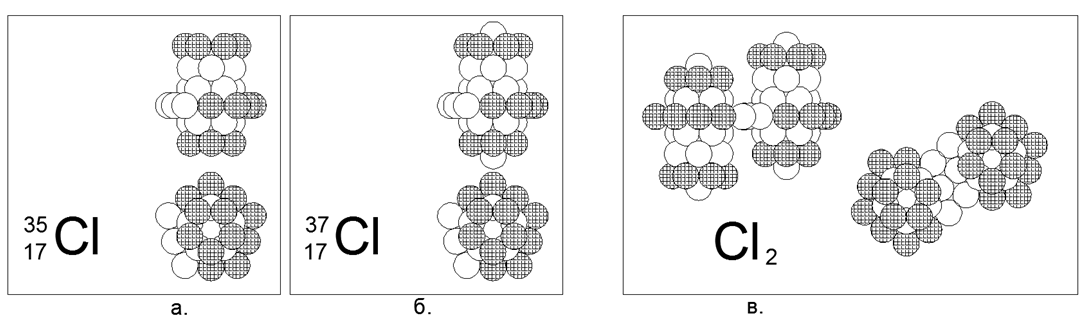
17. Chlorine (17Cl) - has two stable isotopes of 35 and 37 nucleons.
Isotopes have a prevalence of 75% and 25%, respectively. Chlorine is a non-metal and Diatomic Gas under normal conditions. We assume that the main form for the gas is a wide 10-nucleon incompletely charged ring with an embedded cylindrical structure of three 5-nucleon rings along the axis, where the end rings of the tube are fully charged. For the heavy isotope Chlor-37, at the ends of the main cylinder, they are additionally attached one by one nucleon. Chlorine-36 radioactive with T1 / 2 = 301 thousand years. Unstable Chlor-36 is possible both in the variant with a nucleon at the open end of the central tube, and with a hammer into the center of a 10-nucleon ring. With the proximity of the mass numbers of Chlorine and Sulfur, that even the mutual intersection of the numbers in the isotopes (isobars) are present, the structure of the nucleus turns out to be radically different. The difference in the structure of the nucleus also affects the strong differences in chemical and physical properties.
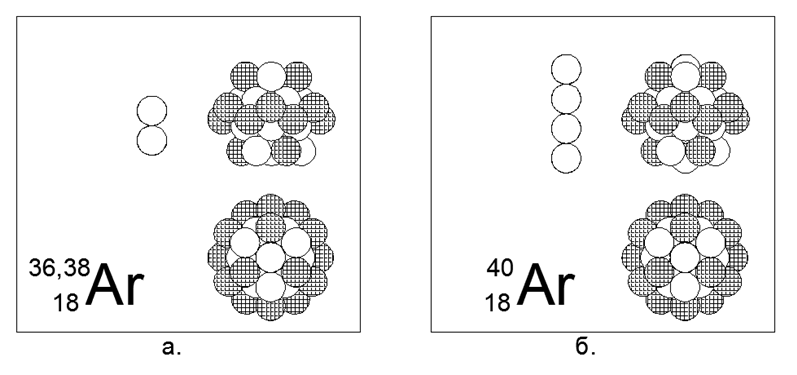
18. Argon (18Ar) - has three stable isotopes of 36, 38, and 40 nucleons.
Isotopes on Earth have a prevalence of 0.337% - 0.063% -99.6%, respectively, although there is a completely different distribution in space. On Earth, all Argon-40 is obtained from decaying radioactive Potassium-40. Argon is an inert gas. It can be assumed that the main form is a cylindrical structure of four pieces of 6 nucleon rings, in the central tunnel of which 2 or 4 additional nucleons are placed in massive isotopes, and a 12 nucleon fully charged ring is put on top of the central tube. A similar Fully Charged Outer Ring is a characteristic feature of Inert Gases, according to the developed Nucleus-Atom theoretical model.
Argon-36 is an isobar to Sera-36 (the second pair in the periodic table), but the structures of the main form are significantly different. It can be assumed that one of the following alkali metals, namely Potassium or Calcium, will be close to Argon in the structure of the core.
The gradual increase in the atomic mass with a linear increase in the number of charged nucleons according to the Periodic Table made it possible to build a consistent number of constructive solutions for the Atomic Structure of Architectural Elements of chemical elements correlated with their physical and chemical properties. Atomic-element models as a mandatory requirement include the ability to build all stable isotopes without a radical change in the appearance of the Atom.
The main conclusion reached at this stage is the following idea:
With all the difference in chemical properties of substances closely located in the table (For example: Inert Gas and the Alkali Metal number following it), they will be structurally different only by the arrangement of charged nucleons on the outer perimeter of the atom or in the inner rows. Thus, a periodic change of properties is associated with a smooth transition of the concentration of charged nucleons from the inner zone of the nucleus to the outer one.
imoninpgd@gmail.com
The Atom model with a large Core, comparable to the size of the Atom as a whole.
At present, in Physics, the Rutherford-Bohr atom model is generally recognized, in which a small heavy positively charged nucleus is surrounded by almost weightless electron shells, while the size of the electron shells is 1000 times larger than the size of the nucleus.
The Rutherford-Bohr atom model has many inherent flaws that have been hushed up for 100 years since its creation.
The only thing that explains the model of the Rutherford-Bohr atom is the experience of dispersing the flow of alpha particles on a thin golden foil. From which a conclusion was drawn about the vanishingly small size of the positive nucleus in the composition of a huge atom filled with the emptiness of the electron shells.
And how does the Rutherford model explain the existence of solids with fixed density?
But she does not explain any properties of real substances, known to us by everyday life.
It was for this reason that I had to compose my own model of the structure of the atom.
None of the existing models can explain everything at once.
I in my model do not explain the dispersion of alpha particles in Rutherford’s experience.
Well, the Rutherford model explains only one experiment on the dispersion of alpha particles on gold foil, but is unable to explain anything else.
But my model explains the existence of solids, liquids, gases and all phase transitions between them.
In the Rutherford-Bohr model, it is completely incomprehensible on what forces there are solid substances that make up all the objects around us, the transition from a solid to a liquid state and from a liquid to a gaseous state of a substance is also not clear.
It is time to try to rethink the Atomic Structure Model and create a new concept that will be able to explain both the Strength of solids and the elasticity of rarefied Gases.
A new Atom model is proposed for consideration, the main features of which are the following theses:
- The nucleus of the atom practically coincides in size with the outer boundary of the atom;
- The nucleus of an atom is not a chaotic lump of nucleons (neutrons and protons), but has a very clear Structure-Architecture, which is responsible for all the physical and chemical properties of a particular simple substance from the Periodic Table;
- Atoms in solids are contacted directly by their solid nuclei;
- The transition to different phases (solid-liquid-gaseous) occurs when the relative position of individual nucleons (or their elements) in the structure of the nucleus changes;
- The concept of the Atomic E-Shell is eliminated from the New Atom Model, and all interactions of Atoms are carried out through direct contact of nucleons of nuclei and known Electrostatic and Magnetic fields attached to specific nucleons in the nucleus.
- There is no separate particle "Electron" in this model of the atom.
The nucleus of the atom practically coincides in size with the outer boundary of the atom. The coincidence of the size of the Nucleus with the outer dimensions of the Atom makes it possible to give explanations to many previously unexplained phase states, that is, solid, liquid, and gaseous substances.
Solid substances in the new concept are explained by solid Atomic Nuclei that are in direct contact with each other. Moreover, their tensile strength is determined by the attraction of individual atoms at the level of short-range Magnetic forces in the atomic nucleus. Well, and the strength for all-round compression is determined solely by the strength of the dense atomic nuclei themselves. All-round compressive strength tends to infinity, which is manifested in the very fact of the existence of giant objects strongly compressed by gravity, such as stars and planets.
The case of one-sided pressure does not create conditions of all-round compression, and therefore the atoms begin to slip over each other under the action of shear forces in the perpendicular direction from the acting compression force. It is precisely the possibility of such a shift that is based on all metalworking technologies (pressing, forging, stamping), when in a narrow gap between the rollers it is possible to roll a metal blank into a very thin foil or between the die and punch it is possible to flatten the blank into a “glass” shaped product. That is how thin-walled beer cans are made of aluminum flat cakes with the help of stamping.
The nucleus of an atom has a very clear and unique Structure-Architecture, which is responsible for all the physical and chemical properties of a particular simple substance from the periodic table.
The transition to the consideration of "small" details of the structure of the nucleus allows you to move from the existing negligible Numerical Characteristics of the number of nucleons and the magnitude of the charge on the periodic table to the informative Qualitative Characteristics of the Architecture of the Nucleus Structure, which should carry the entire set of chemical and physical properties of a simple substance.
The transition to different state of aggregation (solid-liquid-gaseous) is carried out by changing the relative position of individual nucleons in the structure of the nucleus or a change in the nutria of the nucleons themselves.
In the model of the Atom of Rutherford-Bohr, the nucleus was thousands of times smaller than the size of the Atom, and therefore the effect of its tiny nucleus on external phase transformations was simply not considered. With a thousand-fold increase in the nucleus size, its role in the influence on all the physical properties of a substance, including phase transitions to various states of aggregation, dramatically increases. With the direct contact of large multi-nucleon Atomic Nuclei, a new type of interaction arises, while being based on the long-known Magnetic and Electrostatic forces.
At close contact of the Nuclei, enlarged to the outer boundaries of the Atom, the previously ignored phenomenon, “Electrostatic and Magnetic Interaction of Short-Range Order”, that is, the interaction of Strong Magnetic and Electrical Charges of nucleons at distances close to the nucleus size and the size of individual nucleons, begins to manifest itself.
The previous model considered the atom electrically neutral, and the separation of the negative charge of the electron shell and the positive nucleus was not taken into account.
In the new model, the interaction of Nuclei-Atoms can be considered at the level of the local effect of the close convergence of like poles of separated intranuclear Charge-Dipoles. Such an approach makes it possible to create a model of solid matter on the forces of magnetic attraction, as well as a model of static gas states on the forces of electrostatic repulsion. The intermediate state in the model of the Liquid phase has elements of the models of the Solid and Gaseous phase, opposed to each other in a narrow gap between the atoms.
Gas exists on the repulsive forces of electrostatic charges of the same name in the dipoles of individual nucleons, when all the electric dipoles of an atom are turned outward by the same electric pole. At the same time, all the neighboring gas atoms are tangled with the same poles of the electric dipoles (the atom remains totally neutral), which creates their local Electrostatic mutual repulsion. (Fig.1, Fig.2, Fig.3).

Fig.1. The interaction of fields of point charges according to the principle of superposition: a) Two isolated opposite charges with a correct radially directed (spherical) electric field, where the lines of force are evenly distributed and go along radii to infinity, that is, in an abstract model, their fields do not interact; b) The real shape of the force lines of two closely spaced single opposite charges, where, according to the superposition principle, the positive charge force lines are closed on a negative charge, and at infinity the total charge of this system is perceived as zero and the lines of force do not go from the dipole to infinity; c) The real form of the lines of force of two closely spaced single like and one-dimensional charges, where, according to the principle of superposition, the lines of force of the same charges do not intersect, but are displaced into separate half-spaces, and at infinity the total charge of this system is perceived as a point charge of twice the nominal value.
That is, from a distant macro-environment, neutral atoms appear to be uniformly neutral, whereas in the near-order with neighboring atoms, gases are kept in a consistently equidistant state on the forces of the same electrostatic repulsion.
In the “Electrostatic Short-Range Order” model, gas molecules cease to move with enormous speeds, remaining in a state of electrostatic rest, and their temperature is determined solely by the strength of the electrostatic field in the short-range interaction order. A smooth change in the gas temperature is realized by a smooth synchronous change in the length of the arms of the electric Intercranial Dipoles in the radial direction of the atom. The shorter the dipole arm, the shorter the length of the electrostatic corona petals it can create.

Fig.2 Distribution of the power lines of the electrostatic field on the plates of a capacitor with distributed and point charges: a) Flat capacitor with a distributed charge Negative plates (the field does not intersect the plate) and Single point charges on the Plus plate (the field closes outside the gap between the plates) ; b) Spherical capacitor with a distributed charge on the Minus lining and Single point charges on the Plus lining; c) Spherical capacitor with single point charges on the Plus-lining and Single point charges on the Minus-plate (short-circuiting the field on external charges creates long lobes of lines of force that extend far beyond the solid outer spherical plate of the capacitor).

Fig.3. a) View of the electric field of the dipole in the composition of the free nucleon; b) holding on a magnetic force a chain of 6 nucleons closed in a ring, while the magnetic field lines are entirely closed inside the ring and do not go out into outer space; c) The electric field of the dipole of the nucleon ring, which forms the electrostatic Corona (with the petals of the power lines of the electric "crown" going far beyond the limits of the size of the atom).
The electrostatic close interaction of repulsion in gases and the magnetic attraction of nuclei in the liquid (solid) phase well explain the process of vaporization with gradual heating and critical states of vapor on saturation. So evaporation (exit from a solid or liquid phase into a gaseous state) occurs when the atom is heated, the Electrostatic dipoles of the nucleons are turned out by the same poles to the outside to such an extent that at some moment the electrostatic repulsion becomes greater than the magnetic force of neighboring atoms. Condensation occurs in the reverse order: the cooling of the atom leads to a decrease in the strength of the Electrostatic repulsion when the electric dipoles turn to the neutral position.
The work function (energy of vaporization) is the additional energy of the field spent by the atom during the expansion of the electrostatic corona in an empty gas space. The output energy (heat of vaporization) with increasing gas pressure should decrease at equal temperature of liquid and gas above it, which is confirmed by the example of the dependence of the value of heat of vaporization at different air pressures around (see tabular data for pressures below 1 atm. In high mountains). Also, when the pressure and boiling point of water increase (tabular data of the boiling point of Water at different pressures above 1 atm.), The work function decreases, which is explained by the reduction in the size of the intermolecular distances in the liquid and gaseous phases, thereby reducing the volume of the Crown electric field, which additionally absorbs energy at its disclosure after leaving the dense water in the rarefied gas phase.
The transition from the state of steam to the state of ice at negative temperatures must pass through the phase of liquid water. At the same time, on the surface of the already solidified ice, a thinnest layer of the liquid phase of water with a temperature above zero Celsius is formed, in which the heat flux from the folding of the field of condensing water molecules is distributed. The greater the temperature difference between ice and 0 ° C, the thinner the layer of water on the surface, since a large temperature difference increases the intensity of heat exchange in the liquid-ice layer, with a proportional decrease in the thickness of the water layer to conduct the desired heat flux from the condensed water molecule.
The thinnest layer of water on the ice surface when interacting with the support from the external load (the sole of the boot or the sledge runners) creates conditions for the appearance of "hydrocline" even at almost zero relative load velocities relative to ice. The thinnest layer of water with a thickness of several molecular rows simply does not have time to be squeezed out of a thin gap, while the viscosity of liquid water turns out to be very low, which creates the possibility of the sole slipping on ice from a water “hydrocline” lubricant.
If the thickness of hydroclin is related to the temperature difference with respect to 0 ° C, then the ice sliveriness should decrease with increasing frosts. It is this behavior of the Icebreaker that can be traced in practice, when the greatest slip on the roads is noticeable at temperatures around zero Celsius. In severe frosts, when driving on ice, direct contact is solid-solid with almost no formation of hydrocline, as if instantaneous molecular seizure of the ice with the sole (tire) occurs, freezing the ice to the ice.
According to some data, the thickness of the water film on the surface of ice, which is equal at -5 degrees to 100 nm, at -35 degrees decreases tenfold to 10 nm, and at -170 degrees it consists generally of a single layer of molecules. So, the inhabitants of the Arctic say that dragging sleigh on ice at very low temperatures is the same as dragging them across the sand (there is little lubrication in this case).


Fig.4 Schematic representation of the boundary zone of contact of different phases Liquid-Gas (Water-Air) in the case of Electro-Static Theory of the existence of Gaza: a) Gas at a pressure of 1 atm. above liquid water (the distance between the centers of the molecules is approximately 10 times greater than in the liquid phase); b) The same gas above water at the same temperature, but at a pressure of 10 atm., while the distance between the centers of the gas molecules is reduced by 10 ^ 1/3 = 2.15 times. One can clearly see the deformation of the contacting monopolar electric corona of gas atoms when molecules approach each other. The lines of force can not intersect, and therefore are forced to deform, occupying a smaller volume, which leads to an increase in mutual repulsion forces (increase in gas pressure).
Tab.1. Heat of vaporization for water depending on pressure and temperature.


The existing equations of state of an ideal gas (Boyle-Marriott law) well describe gases at low pressures (normal conditions) and high temperatures. But at high pressures, this ideal law does not allow one to describe transitions to either a liquid or, moreover, to a solid state.
The van der Waals law tries to correct inconsistencies by introducing additional factors, fitting the theoretical curve to the experimentally derived relationship. In this case, there is a hidden rejection of the Rutherford-Bohr atom model, since in the van der Waltz equation, the atom is represented by a solid ball that fills the entire volume at its outer boundaries.
In this case, the nature of the interaction of the atoms of the balls is not qualitatively explained, leaving explanations at the level of weightless statistical electron shells over the tiny nucleolus, as it should be according to the Rutherford-Bohr model.
In the case of the proposed model, where repulsion of like-charged surfaces occurs, it becomes possible to consider an Understandable Static Model, where each element of the model has a clear physical embodiment and physical meaning.
So the interaction of dipoles is expressed by the simplest formula of direct pairwise interaction for four point charges assembled in pairs in mechanically strong dipoles with an arm L and the distance R between the proximal ends of the dipoles:

F = K q2 (1 / R2 - 2 / (R + L) 2 + 1 / (R + 2L) 2)
Having led to a common denominator and opening the brackets in the numerator, we get an even more cumbersome expression:
F = K q2 (2R2L2 + 12RL3 + 4L4) / (R2 (R + L) 2 (R + 2L) 2)
A feature of the computational model for dipoles is that the repulsion function of the dipoles rushes to Infinity away from the center of the atom, or rather at its outer edge, where direct contact of atoms is possible when exposed to the most powerful forces of magnetic atomic attraction. It is this kind of contact between two forces that vary sharply in magnitude and opposite in sign on the atomic border and allows us to create a model of balanced Repel-Attraction of atoms with each other.
If R >> L, the function of electrostatic repulsion of dipoles is simplified to the form:
F = K q2 2L2 / R4
Magnetic attraction has a saturation property and the graph of the function does not go to infinity even with direct contact of atoms, so Magnetic attraction will always find an equilibrium point with Electrostatic repulsion on the steep hyperbolic slope of the Electrostatic characteristic.
Of course it is clear that in reality there are no infinitely large forces, but there is a certain limit of maximum values (for example, the attractive forces of atoms can be estimated by direct measurement of the tensile strength in a single crystal of a substance, and the finiteness of the electrostatic interaction forces is determined by the fixed energy during the electron annihilation with Positron). When two atoms approach each other, the Repulsive Forces at some point also cease to grow rapidly and reach a certain fixed final value. Thus, two not infinitely large values of attraction and repulsion balance in a certain point of mutual equilibrium, which can be found at the intersection of the graphs of these two functions, when plotting graphs in one coordinate system. (see Graph 1)

Graph 1. Graphs of the active forces of magnetic interatomic attraction (a) and electrostatic repulsion forces at different temperatures: in solid matter (b); at melting T (c), with molten liquid state (g) with an interatomic gap (g "); critical vapor-liquid equilibrium temperature (e) with a touch of repulsion curves and attraction at point (d "); superheated steam (e). The R axis is the distance between the surfaces of the outer boundaries of the nearest contacting atoms. At the same time, the area of the closed segment between the curves (r-a) is equal to the output energy (detachment) of the atom from the liquid phase array, and the region to the right of the intersection of the repulsion curves with attraction is the gas phase region, where the area under the repulsion curve to the attraction curve is equal to the field work energy during vaporization (field expansion after separation from liquid) at given temperature (one of the letter curves) and gas pressure (R max is the distance between gas molecules at a given pressure).
The van der Waals equation draws a very similar picture, but does not explain the physical meaning of the forces of attraction and repulsion, while slyly pushing the zone of infinite asymptotic growth of the function into the inaccessible zone of the center of the atom.
The liquid state of a substance is the moment when the heating of the Electrostatic dipoles to the repulsion has already begun when heated, but this repulsion is not yet able to break the bond of the magnetic attraction of the atoms. As a result, a small gap appears between the atoms (g ”in Graph 1), leading to a sharp increase in the mutual mobility of the atoms on the layer of electromagnetic“ lubricant ”.
The solid-gas boundary layer is formed when a magnetically coupled solid substance comes into contact with electrostatically stressed gas atoms. Gas atoms cannot repel themselves from a solid substance with unopened electrostatic coronas of atoms and are pressed to a solid surface. This forms the boundary layer of Gaza atoms pressed to the surface of a solid substance.At the same time, the boundary gas layer is still capable of pushing away from itself other gas atoms, since the boundary gas layer remains electrostatically stressed to the necessary degree. Such pressing of the boundary layer of gas (air) into a solid metal explains the almost instantaneous appearance of metal oxides on the surface. Further contact of the boundary layer of the gas is already carried out with a solid protective film of oxides on the metal surface, which protects the underlying layer of pure metal from further oxidation.
Modeling alleged Structures-Architectures of chemical elements. In the future, the proposed models of the structure of the nuclei of atoms of various substances are considered, where the metal ball with strong magnetic properties will act as a nucleon (neutron, proton). The magnetic interaction of nucleons meets the criterion of the Non-centrality of the force interaction of nucleons in the nucleus and the saturation of these forces when nucleons approach each other, which has long been assumed in Nuclear Physics for strong nuclear interactions.
Modern rare-earth magnets have a very high magnetic force, which makes it possible to build plausible models of atomic structures, relying only on the magnetic holding forces of the Sharik-Nucleons as part of a single core with a unique Architecture. At the same time, the magnetic forces themselves act as Critics Controllers for each such model of nuclei, since not all spatial combinations of balls can be assembled in a stable form due to the mutual attraction-repulsion of polarly magnetized balls.
The model of each atom is assembled from the number of nucleons given by the periodic table (including all stable isotopes). Reconstructions of models are created from neodymium magnetic balls with a diameter of 5 mm. Currently, these magnetic balls are sold as puzzle toys called Neokub, which includes 216 magnetic balls of the same size (cube 6x6x6 pcs.).
Based on the obtained geometric features of the atomic models of each simple substance, we will try to find logical connections between the shape of the atomic model and the properties of the real substance at the macro level.
At the initial stage of the simulation of nuclear structures, some regularities emerged. So the magnetic balls tend to line up in chains, and quite long chains easily close into rings. Ring magnetic structures are very strong and stable, while the external magnetic field of these rings is sharply reduced, since almost all the magnetic lines of their magnetic flux separate magnetic balls are closed through adjacent balls of their magnetic nucleon ring (see Figure 5). Rings of the same size (an equal number of nucleon balls) are able to easily connect with each other, forming very stable columnar structures that can grow almost infinitely along the length with new commensurate rings (Fig.6).
The chains of nucleon balls can be connected in parallel to each other in two versions:
- associated magnetic flux directions
- backward direction of magnetic flux
The passing direction of the magnetic fluxes gives a more solid and compact connection of chains of balls (the balls are located at the vertices of isosceles triangles). Counter-directed magnetic fluxes cause the balls to be located on the tops of the squares, which gives a less dense and less durable packaging of nucleon balls

Fig.5. Options for the interaction of magnetic balls in the simplest versions:
but. Linear column of 3 magnetic balls (steady);
b. An annular closure of the magnetic flux itself on itself from 3 balls (steady);
at. Кольцевое замыкание магнитного потока самого на себя из 2-х шаров (неустойчивое), при малейшем сдвиге перестраивается в столбовой вариант по типу (а.);
г. Развёртка замкнутого в кольцо магнитного потока одиночной цепочки шаров- нуклонов;
д. Развёртка замкнутого в кольцо магнитного потока сдвоенной цепочки шаров- нуклонов с попутным расположением магнитных потоков, при этом шары расположены по вершинам равносторонних треугольников, что обеспечивает максимально плотную и жёсткую к сдвигу пространственную упаковку;
е. Развёртка замкнутого в кольцо магнитного потока сдвоенной цепочки шаров- нуклонов со встречным расположение магнитных потоков, при этом шары расположены по вершинам квадрата, что обеспечивает не самую плотную и нежёсткую к сдвигу пространственную упаковку. При встречных магнитных потоках в случае сдвига цепочек связи между рядами резко слабеют.

Рис.6. Магнитостабильные Столбы из 6-нуклонных магнитных колец:
but. Трёхрядный столб с противоположно направленными магнитными потоками в кольцах;
b. Трёхрядный столб с попутно направленными магнитными потоками в кольцах.
Попутное соединение магнитных потоков с круглыми магнитами-шариками оказывается более прочным. На видах сверху в центре 6-ти нуклонных магнитных колец виден просвет, куда свободно помещается по одному шарику в каждом кольцевом ряде.
.
In addition to combining one-dimensional rings, it is possible to join and different-sized rings in various combinations, giving a variety of spatial forms. It is precisely this variety of possible forms of combining ring magnetic structures and individual magnetic balls that makes it possible to create many models of the Architecture of nuclei with a similar number of nucleons, but related to different substances with radically different chemical and physical properties.
As is known, a set of isotopes with different numbers of neutrons in a composition can correspond to a single chemical element. Thus, the Pillar structures of atoms from rings with six or more nucleons have a lumen in the center, which allows placing additional nucleons inside the column without changing the appearance of the atomic nucleus. (Fig.6.b).
Based on the proposed version of magnetic attraction and electrostatic repulsion, it can be assumed that metals include nuclear structures that have an additional outer ring of magnetically coupled nucleons on top of a central column of one-dimensional nucleon rings (Fig. 7). This assumption is based on the fact that the external magnetic ring is not charged, thereby increasing the gap between the internal electro-charged column when approaching other atoms of the same type. Such a gap raises the boiling point and melting point of the metal, as well as creates a wider area for the liquid state zone.
Substances that are Gases under normal conditions, on the contrary, should have a charged nucleon ring in the outer loop, providing electrostatic repulsion from gas atoms of the same type, while a large number of nucleons can be placed inside the charged ring, creating a wide isotope series of this element.

Fig.7. Возможные Магнито-Стабильные Варианты соединения столбовых структур с одноразмерными ( верхний ряд — неметаллы) и разноразмерными ( нижний ряд- металлы) кольцами и с различными вариантами заполнения центральных просветов для различных вариантов нуклонного состава. Рядом с рисунками основной кольцевой конструкции указано количество шариков, свободно размещаемых внутри просвета магнитных колец, с получением диапазона изотопов к данной основной конфигурации. На рисунке показаны далеко не все возможные конфигурации атомных магнитосцепленных структур, так как разнообразие всевозможных комбинаций разнонуклонных колец крайне велико, что не позволяет их все показать на одном маленьком изображении.

Рис.8. Взаимодействие магнитных колец при «точечном» контакте атомов с образованием межатомной связи магнитного притяжения:
but. Магнитный поток в 6-ти нуклонном кольце в свободном состоянии;
b. Искажение магнитных линий в 6-ти нуклонных кольцах в месте внешнего касания с образованием химической связи на притяжение при попутном магнитном потоке колец в точке касания, контакт максимально жёсткий и плотный с касанием в двух точках, такая химическая связь наиболее прочная;
at. Искажение магнитных линий в 6-ти нуклонных кольцах в месте внешнего взаимного касания с образованием химической связи на притяжение при встречном магнитном потоке колец в точке касания. Контакт нуклонов происходит в одной точке, а потому связь подвижнее и слабее, чем при попутном контакте, при этом связь стремится трансформирватся в двухточечную устойчиву (рис.г).
г. Искажение магнитных линий в 6-ти нуклонных кольцах в месте внешнего взаимного двухточечного касания при встречных магнитных потоках. Контакт нуклонных колец происходит в двух точках, а потому связь достаточно прочная.

Рис.9. а) Вид неполной электростатической Короны одиночного атома вещества, которое встречается в газообразном виде только как Двухатомный газ ( N2, О2 и т.д.),; б) вид полной электростатической Короны двухатомного газа, при заполнении дыры в электрической короне одного атома неполной Короной другого такого же атома вещества.
Синтез магнитных структур Ядер-Атомов
In the following, we will look in detail at the most likely Structures - the Atomic Architecture of individual elements with reference to the features of their nucleon composition and known physical properties (density, strength, Tflav, Tcip, etc.).
The following table shows the 1-2-3 periods (short) of the periodic table, where instead of the fractional atomic weight indicated are the integer weights of individual stable isotopes found in nature (Table 2).
Tab.2. Atomic weights of stable isotopes of chemical elements of the 1-2-3rd periods of the periodic table.

In the 1st period and the 2nd period (the first full row of the table) of the periodic table contains elements with a small number of nucleons. Small-sized atoms of these periods allow us to consider such spatial models, which correspond to unique forms that can set the direction for constructing models of atoms in all subsequent periods (See Figure 7). Moreover, the most reasonable variant of modeling can be considered a gradual increase in the length of the main charged ring of the Atom, complementing it to the maximum nucleon composition with external (metal) and intra-ring nucleons (non-metal) in accordance with their chemical status, and then looking for an analogue according to the table of nucleon correspondences of chemical elements .
1. Hydrogen (11H) 1proton (1p). The free substance has the form H2.
The construction of the hydrogen atom does not require any effort, since it consists simply of a single proton ball. Because of the open magnetic flux, monoatomic hydrogen is extremely active, which causes it to react with the nearest free substances or contact other Hydrogen atoms to a stable closed ring state.
Deuterium (21D) 1 proton + 1 neutron (1p + 1n) - the form is also unique in the form of two stuck balls.

2. Helium (42He) 2 protons + 2 neutrons (2p + 2n)
For Helium, it is already possible to build several variants of the nuclear structure models:
- Linear - four nucleons in a row,
- Flat (Square) - four nucleons in the corners of a flat square,
- Spatial (Tetrahedral) - four nucleons in the corners of a volume polyhedron-Tetrahedron.
In this case, of the 4 magnetic balls, it is possible to build only the flat Square shape of the nucleus. The tetrahedral form on the magnetic forces is not held, turning immediately into a square.
In the future, we will focus only on the spatial forms of atoms, since linear variants in more massive atoms cannot give any meaningful incarnation.
Rare isotope Helium (32He) 2 protons + 1 neutron (2p + n)
In this configuration, only a flat triangle is possible.
3. Lithium (73Li) -7 nucleons (3p + 4n)
For Lithium, it is possible to create both a flat and a bulk model of an atom.
Plane model is a hexagon with one nucleon in the center. A volumetric model is a flat pentagon with two balls at the poles (Poles are points at the ends of the axis of rotation of the main axisymmetric part of the atomic nucleus). Both models have a pronounced star-like symmetry. Lithium is a metal, very light (0.534 g / cc), relatively fusible (Mp = 454K). The rare stable isotope Lithium (63Li) -6 nucleons (3p + 3n) can be modeled in both forms, so the choice in favor of any model cannot be made.

4. Beryllium (94Be) - 9 nucleons (4p + 5n).
The only stable long-lived isotope. There is a relatively long-lived isotope Beryllium (104Be) - 10 nucleons (4p + 6n) with a half-life of 1.4 billion years. Many implementations are possible. The most stable and compact form of sensations looks like a star-shaped form of two triangles connected by planes to a prism, where one side of the triangular prism is attached to the side faces. Beryllium is a light metal (1,848 g / cc), relatively refractory (Tpl = 1551K). In the future, the STARNESS will be traced in most metals. When the 10th ball is added to the resulting structure, the form itself is modified into another star shape, but asymmetrical in the plane. A new form can be described as a six-pointed star with a filled center, with a triangle of nucleon balls attached to its side plane.When viewed along the axis of the "star" at both forms of Beryllium isotopes, a single-type three-pointed star is clearly visible, determining the basic external properties of a simple substance.

5. Boron (115Be) - 11 nucleons (5p + 6n).
Stable long-lived isotopes 10B and 11B. The spatial configuration of 10V consists of two pentagonal rings connected parallel in the passing or opposite direction of the magnetic flux. The spatial configuration 11B repeats the structure 10B, only one neutron is added along the axis of the rings.

Thus, it can be assumed that a non-metal differs from a metal in its basic external shape: a non-metal is a cylinder of similar rings, a metal is some star-shaped (disk-shaped) shape.

6. Carbon (126C) - 12 nucleons (6p + 6n).
Stable long-lived isotopes 12C (98.93%) and 13C (1.07%), as well as radioactive 14C. An extremely interesting element that is very common and has an infinite variety of structural forms in nature (Graphite, diamond, coal, soot, nanostructures-fullerenes, etc.) This form-content requires some amazing special properties from the structure of 12 balls. Such a structure can be a flat, non-equilibrium hexagon with three balls in the center.
The charged nucleons are arranged in a triangle, on the sides of which three pairs of neutrons are attached. This results in three-beam symmetry, where external neutrons are located on the symmetry axes, ready for the magnetic coupling of other atoms. Of such hexagons, Layers of Graphite, or the surface of nanotubes and three-dimensional structures of closed Fuleren structures, are easily laid out.

Fig. View of a layer of graphene (graphite) composed of hexagonal flat C12 carbon atoms.
In the series of non-metals, those substances that, under normal conditions, are gases, stand out. These are monatomic Inert (noble) gases, atmospheric diatomic Nitrogen and Oxygen, and active diatomic halogen Fluorine.

7. Nitrogen (147N) - 14 nucleons (7p + 7n).
Stable long-lived isotopes 14N and 15N. In its free state under normal conditions, Nitrogen is a diatomic gas, and it is very little active. 78% of nitrogen is the atmosphere of our planet. To possess the property of gas at N.u. the atom is required to have charged nucleons on the outer perimeter, without discontinuities for pair neutrons. But since the gas is only a diatomic molecule, it means that in the structure of the atom there is a magnetoactive segment of two or three neutrons in a row on the outer border, which are the atoms of the Nitrogen in the N2 molecule. Such a form of 14 atoms is easily assembled in the form of a nonequilibrium hexagon, similar to a rhombus, where you really eat a site with three neutrons in a row on the outer perimeter of the atom. The bond in the N2 molecule is so strong that the gas remains in a molecular state even at 5000 degrees C.

8. Oxygen (168O) - 16 nucleons (8p + 8n).
Stable long-lived isotopes 17О and 18О. The estimated form of the most common (99.7%) isotope 16O is a 5-square of ten nucleons (8 charged, 2 neutral), with a pronounced magnetic neutral-magnetic contact space in the outer ten-atom ring. The inner liner consists of a 5 nucleon ring with an additional sixteenth nucleon in the center. 10 and 5 nucleon rings can not be joined in a plane, and therefore create a spatial dome-shaped form. Isotopes are created by the capture of additional neutrons in the center under the "dome". The presence of an external two-nucleon gap in the ring ensures the creation of a diatomic O2 molecule. The bond strength inside the O2 molecule is much weaker than that of Nitrogen, and therefore oxygen is much more active and enters into oxidation reactions (burning) at sufficiently low temperatures.

9. Fluorine (199F) - 19 nucleons (9p + 10n).
Only one stable isotope, unstable live from fractions of seconds to units of hours. The assumed form of the structure: a flat hexagon of nested 12 and 6 nucleon rings and the 19th nucleon in the center. The lack of charged nucleons in the outer ring creates a magnetic attraction region of three neutral nucleons, allowing the creation of a diatomic F2 molecule, after which the substance exhibits the properties of a gas under normal conditions. Fluorine-2 is a cryogenic gas, that is, it becomes liquid only at extremely low temperatures (85K or -188C), which makes it related to the properties of the two previous diatomic gases.

10. Neon (2010He) - 20 nucleons (10p + 10n) content in nature of 90.47%.
Stable long-lived isotopes 21He (9.25%) and 22He (0.27%). Estimated nucleus structure: 10-nucleon charged outer ring plus two inner nested 5-nucleon rings.
Isotopes 21 and 22 are created by adding one nucleon to the center of one of the 5 nucleon rings along the axis.
In Table 1, substances with a single stable isotope are of the greatest interest, since their Unique structure should not allow one to accept additional nucleon balls without introducing distortions into the external form. Such substances include Metals Beryllium-9 (Figure 7) and Sodium-23 (Figure 6), Aluminum-27, as well as non-metal Fluor-19, Phosphorus-31.

11. Sodium (2311Na) - 23 nucleons (11p + 12n) - This is the only stable isotope in nature, which allows you to choose a unique nucleon configuration for it.
Of the 23 balls, only one version of the dense, symmetric magnetically stable star-disk structure was created. A ring of 10 nucleons is wound around the central part of two laminated planes of 5-nucleon rings. Inside the central part between two 5-nucleon rings, the central 21st nucleon is driven in. From the central part along the central axis one more nucleon is attached (the 22nd and 23rd). In general, the core looks like a yula in the form of a convex lentil. Due to the fact that 5-nucleon and 10-nucleon rings can not line up in a plane, the shape of a 10-nucleon ring acquires a zigzag shape, while the inner 5-nucleon rings are somewhat apart, which allows 21- to fit in the center between them. nucleon.
12. Magnesium (12Mg) - 24, 25, 26 nucleons.
Isotopes have a prevalence of 78.6% - 10.1% -11.3%, respectively. Thus, it is obvious that the main form is a disc-shaped structure of two 6-nucleon rings in the central part and a 12-nucleon ring, wound around. Isotopes are created by driving one or two neutrons into the central tube of a disk, which in a 6-nucleon ring exactly corresponds to the size of a ball-nucleon.
13. Aluminum (2713Al) - 27 nucleons (13p + 14n) - This is the only stable isotope in nature, which allows you to choose a unique nucleon configuration for it.
Of the 27 balls, we managed to create an axisymmetric version of a dense magnetically stable star-like structure with a multiplicity of rings of 5 nucleons: a central column of three 5 nucleon rings and an outer 10 nucleon ring, and two nucleons at the ends of the central tube complete the picture. Isotopes 28 and 29 have a half-life of 2 minutes and 6 minutes, respectively. Isotope 26 - has T1 / 2 = 717 thousand years., And naturally decays into stable Magnesium-26 by electron capture (beta capture). The continuous synthesis of Al-26 takes place in the atmosphere when cosmic fast Protons collide with argon atoms.

14. Silicon (14Si) - 28, 29, 30 nucleons.
Isotopes have a prevalence of 92.2% - 4.7% -3.1%, respectively. Silicon is a non-metal. Under normal conditions, silicon exists in various forms, and all of them are solid substances. In crystalline form, silicon is a semiconductor, which makes it related to carbon from the previous period.
For 28 nucleons, there were no regular forms from a cylindrical row, which made it possible to search for a possible model of an atom in a series of polyhedral plates, such as Carbon and Nitrogen. So 28 nucleons are obtained from two planar hexahedral nitrogen atoms connected by planes.

15. Phosphorus (3115P) - 31 nucleons.
The only stable isotope. Non-metal. Estimated structure: Four 5-nucleon rings in the central tube and a 10-nucleon outer ring in the middle of the central tube, which gives 30 nucleons, and 31 nucleons are hammered into the center of a 10-nucleon ring, where a cavity arises due to non-flat joining 10 -ti and 5 nucleon rings. The charges are arranged in 5 pieces at the ends of the central tube and five more evenly around a 10-nucleon outer ring.
16. Sulfur (16S) - has four stable isotopes of 32, 33, 34 and 36 nucleons.
Isotopes have a prevalence of 95.013% - 0.75% -4.215% -0.017%, respectively. Sulfur is non-metal. We assume that the main form is a cylindrical structure of five pieces of 6 nucleon rings, in the central tunnel of which additional isotopic nucleons are placed. For the rare Sulfur-36 isotope, the structure becomes poorly understood, since two additional nucleons hardly fit inside the central tube. Sulfur-36 not only completely fills the inner tube of the cylinder, but also sticks out with nucleons beyond the boundaries of the cylindrical outer part. This configuration gives the typical non-metallic properties of the substance, that is, with relatively moderate melting and boiling points close to normal conditions.

17. Chlorine (17Cl) - has two stable isotopes of 35 and 37 nucleons.
Isotopes have a prevalence of 75% and 25%, respectively. Chlorine is a non-metal and Diatomic Gas under normal conditions. We assume that the main form for the gas is a wide 10-nucleon incompletely charged ring with an embedded cylindrical structure of three 5-nucleon rings along the axis, where the end rings of the tube are fully charged. For the heavy isotope Chlor-37, at the ends of the main cylinder, they are additionally attached one by one nucleon. Chlorine-36 radioactive with T1 / 2 = 301 thousand years. Unstable Chlor-36 is possible both in the variant with a nucleon at the open end of the central tube, and with a hammer into the center of a 10-nucleon ring. With the proximity of the mass numbers of Chlorine and Sulfur, that even the mutual intersection of the numbers in the isotopes (isobars) are present, the structure of the nucleus turns out to be radically different. The difference in the structure of the nucleus also affects the strong differences in chemical and physical properties.

18. Argon (18Ar) - has three stable isotopes of 36, 38, and 40 nucleons.
Isotopes on Earth have a prevalence of 0.337% - 0.063% -99.6%, respectively, although there is a completely different distribution in space. On Earth, all Argon-40 is obtained from decaying radioactive Potassium-40. Argon is an inert gas. It can be assumed that the main form is a cylindrical structure of four pieces of 6 nucleon rings, in the central tunnel of which 2 or 4 additional nucleons are placed in massive isotopes, and a 12 nucleon fully charged ring is put on top of the central tube. A similar Fully Charged Outer Ring is a characteristic feature of Inert Gases, according to the developed Nucleus-Atom theoretical model.
Argon-36 is an isobar to Sera-36 (the second pair in the periodic table), but the structures of the main form are significantly different. It can be assumed that one of the following alkali metals, namely Potassium or Calcium, will be close to Argon in the structure of the core.
Subtotals.
The gradual increase in the atomic mass with a linear increase in the number of charged nucleons according to the Periodic Table made it possible to build a consistent number of constructive solutions for the Atomic Structure of Architectural Elements of chemical elements correlated with their physical and chemical properties. Atomic-element models as a mandatory requirement include the ability to build all stable isotopes without a radical change in the appearance of the Atom.
The main conclusion reached at this stage is the following idea:
With all the difference in chemical properties of substances closely located in the table (For example: Inert Gas and the Alkali Metal number following it), they will be structurally different only by the arrangement of charged nucleons on the outer perimeter of the atom or in the inner rows. Thus, a periodic change of properties is associated with a smooth transition of the concentration of charged nucleons from the inner zone of the nucleus to the outer one.
Source: https://habr.com/ru/post/440848/